Rep:Llt15 TS 3
Exercise 3: Diels-Alder vs Cheletropic
The cycloaddiiton process between xylylene and sulfur dioxide is possible to produce three different outcomes via Diels-Alder reaction and cheletropic reaction as shown in figure 1:
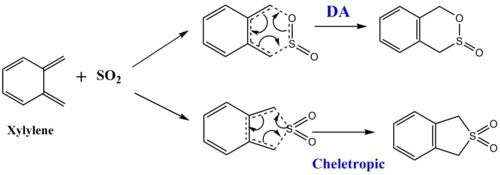
(In this introduction it would be good to explain that you are exploring two Diels-Alder paths Tam10 (talk) 09:56, 30 October 2017 (UTC))
Method used for exercise 3:
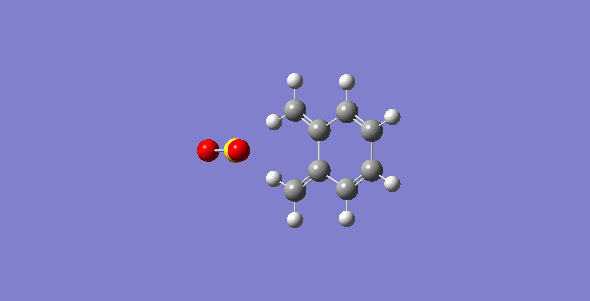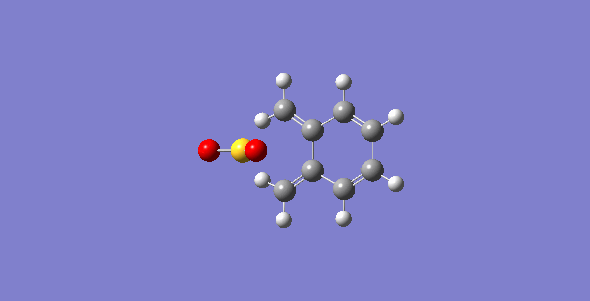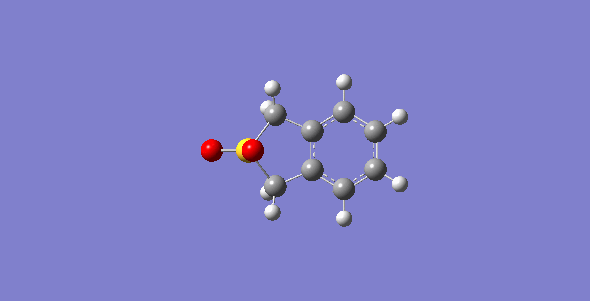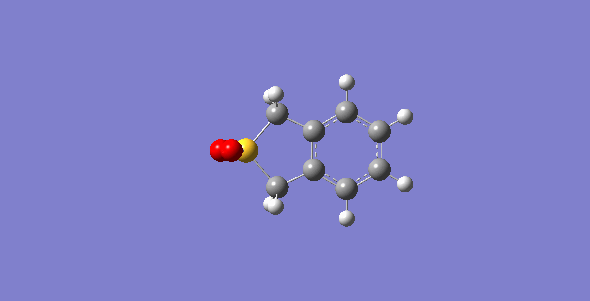
First of all, the exo-DA adduct is drawn and optimized at PM6 level. Secondly, the bonds between xylylene and SO2 are broken and the resulting structure are optimized at PM6 level with the C-O separation set at 2.0 Å and the C-S at 2.4 Å. Then, the bonds are frozen and optimized to a minimum to give a structure closed to the desired transition state followed by a TS calculation. The optimized TS is ran for the IRC calculation and its frequency is checked to make sure it is the correct TS. The optimized TS from previous steps is used to run a second IRC calculation with the geometry of SO2 being changed to make the endo-DA adduct. The endo-DA adduct obtained form the IRC calculation is then optimized to a minimum to get a structure with more accurate geometry. Similar process is done for the cheletropic process starting from the product to obtained the TS. The optimized structures are shown in table 1 and 2.
| Reactants | |||||
|---|---|---|---|---|---|
| Xylylene | Sulfur Dioxide | ||||
(Good, your Jmols show that there are no imaginary frequencies in your reactants Tam10 (talk) 09:56, 30 October 2017 (UTC))
| States | Diels-Alder Reaction | Cheletropic reaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Exo pathway | Endo pathway | ||||||||
| Transition State | |||||||||
| Products | |||||||||
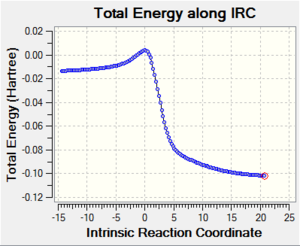
Visualise the reaction coordinate with an IRC calculation for each path. Include a .gif file in the wiki of these IRCs.Xylylene is highly unstable. Look at the IRCs for the reactions - what happens to the bonding of the 6-membered ring during the course of the reaction
Note that xylylene is formed from the pyrolysis of xylene.
Hückel's rule states that for the compounds which are planar and have a contiguous, cyclic array of p-orbitals perpendicular to the plane of ring:
- Those with 4n + 2 π electrons display special stability = aromatic
- Those with 4n π electrons display special instability = anti-aromatic
Where n is an integer starting from zero[1]
Xylylene has a planar structure with conjugated double bond. However, xylylene is overall a non-aromatic molecule as it is not cyclic. By considering only the 6-membered ring, the ring system posses unusual instability due to its anti-aromaticity as it has 4 π electrons. The anti-aromatic character of the 6-membered ring results in a higher energy π electron system and therefore it is highly unstable.
(I'm not sure what you mean here by "xylylene is not cyclic", and why this means it's non-aromatic. It can either have 4 π electrons in the ring, or 6 π electrons and a diradical (highly unstable) Tam10 (talk) 09:56, 30 October 2017 (UTC))
All the three reaction profiles in table 3 depict that all the 3 reactions are highly exothermic indicating the xylylene is highly reactive in order to regain its aromaticity. The IRC gif files show how the double bonds appear to be cyclic and completely conjugated when the 2 new sigma bonds are forming. Along the reaction coordinate, the π electrons quickly rearrange to form a cyclic system and the aromatic stabilisation is acquired from the resonance of the benzene ring structure. All the 3 products are aromatic as each of them consist a benzene ring structure with 6 π electrons (4n + 2 π electrons, obey Hückel's rule). The measurement of the bond lengths of the 6-membered ring structure of xylylene and the 3 products is listed below:
Xylylene:
C-C bond length: 1.46 Å, 1.47 Å, 1.49 Å
C=C bond length: 1.35 Å
Exo/Endo Adduct C-C bond length: 1.47 Å
Cheletropic Adduct C-C bond length: 1.47 Å
All the bonds in the 6-membered ring system of the 3 products have the same bond length (1.47 Å) which is an intermediate value between the average C-C (1.54 Å) and average C=C (1.34 Å) bond length. This can be explained by the delocalisation of π electrons in the ring system and the overall structure is an average from all the resonance form of benzene. So, the alternating single bonds have partial double bond character and the double bonds are weaken as the π electrons cloud is no longer localised along the C-C double bonds.
Calculate the activation and reaction energies (converting to kJ/mol) for each step as in Exercise 2 to determine which route is preferred.Draw a reaction profile that contains relative heights of the energy levels of the reactants, TSs and products from the endo and exo Diels-Alder reactions and the cheletropic reaction
| Measurement | Energies / kJmol-1 | ||
|---|---|---|---|
| Diels-Alder Reaction | Cheletropic reaction | ||
| Exo | Endo | ||
| xylylene | + 468.25 | + 468.25 | + 468.25 |
| Sulfur dioxide | - 313.04 | - 313.04 | - 313.04 |
| Sum of reactant energy | + 155.21 | + 155.21 | + 155.21 |
| TS | + 241.67 | + 237.69 | + 260.00 |
| Product | + 56.31 | + 56.95 | + 0.0105 |
| Activation barrier (Eact) /kJmol-1 | + 86.46 | + 82.48 | + 104.79 |
| Reaction energy /kJmol-1 | - 98.90 | - 98.26 | - 155.20 |
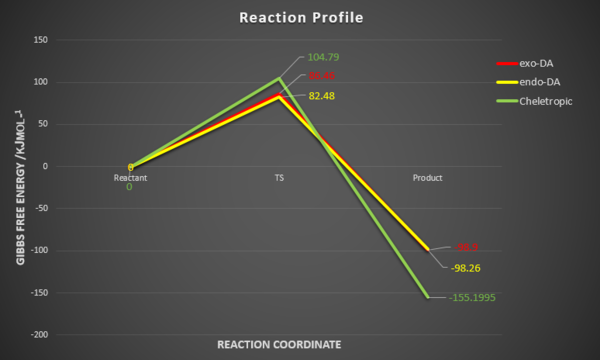
From both the table 4 and figure 9,
In terms of thermodynamic, the cheletropic reaction is the most exothermic reaction. Therefore, the cheletropic product is the most thermodynamically favourable product, followed by the exo-DA adduct then the endo-DA adduct as it yields largest reaction energy.
In terms of kinetics, the endo-DA adduct is the most favourable product, the next is the exo-DA adduct followed by the cheletropic product. This is because this reaction pathway requires the least activation energy to reach its transition state and hence the endo-DA adduct is formed faster.
Conclusion:
The change in bond order due to the change in hybridisation affects the bond length. For example, the decrease in bond order will lead to the formation a weaker, longer bond. For the cycloaddition of 1,3-butadiene and ethylene, the bond formation is concerted and therefore synchronous and the reaction is thermally allowed. Cyclohexadiene and 1,3-dioxole has an inverse electron demand in both exo and endo pathway. From the study of the thermochemistry, the endo-adduct is more kinetically and thermodynamically favourable due to the stabilising effect from the secondary orbital interactions on its transition state despite of the insignificant steric effect. Xylylene and sulfur dioxide can form three different products via the exo/endo-DA reaction pathways and cheletropic reaction. The endo-DA adduct is the kinetically favoured product whereas the cheletropic product is the thermodynamically favoured product.
Additional Information
For exercise 1, go to https://wiki.ch.ic.ac.uk/wiki/index.php?title=Llt15_TS
For exercise 2, go to https://wiki.ch.ic.ac.uk/wiki/index.php?title=Llt15_TS_2
| Diels Alder Reaction | Cheletropic Reaction | |
|---|---|---|
| Exo-pathway | Endo-pathway | |
| File:DA Q3 LLT15 PM6 EXO IRC REDO2.LOG | File:DA Q3 LLT15 PM6 IRC ENDO.LOG | File:CHE Q3 LLT15 PM6 IRC.LOG |
References
- ↑ Jonathan Clayden, Nick Greeves, Stuart Warren, Organic Chemistry, 2nd Ed., OUP Higher Education Devision; Chap 7, pp.161-162