Rep:Llt15 TS 2
Exercise 2: Cycloaddition of Cyclohexadiene and 1,3-Dioxole
Diels Alder (DA) reaction is a streospecific [4+2] pericyclic reaction with its reactivity depending on the relative energies of frontier molecular orbitals. The frontier molecular orbital (FMO) theory[1] simplifies the reactivity of the Diels Alder reaction by assuming the chemistry of conjugated π systems is mostly determined by the interactions between the HOMO of one species and the LUMO of the other. When both the diene and dienophile are cyclic, it is possible to form 2 different products by going through the exo and endo pathway respectively.
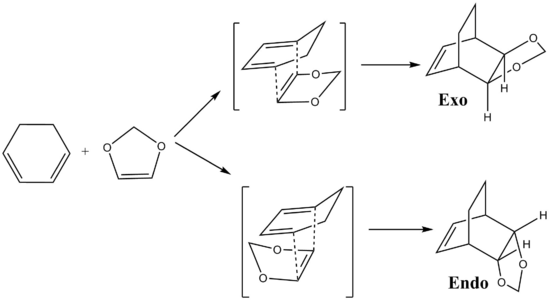
The Diels Alder reaction between the cyclohexadiene and 1,3-Dioxole is studied by firstly optimizing their geometries using the semi-empirical method (PM6 basis set) followed by the DFT method (B3LYP631Gd basis set). Similar as exercise 1, the guess TSs are constructed from the optimized reactant. Different orientations of the 2 reactants facing each other lead to exo and endo-DA pathway respectively in the transition state. The guess TS structures are optimized at PM6 level and then reoptimized again with DFT (B3LYP631Gd basis set) method. After that, the optimized TSs are ran for IRC calculation to obtained the desired exo and endo-products. The results are shown in table 1 and 2.
| Reactants | |||||
|---|---|---|---|---|---|
| Cyclohexadiene | 1,3-Dioxole | ||||
| States | Diels Alder Reaction | |||||
|---|---|---|---|---|---|---|
| Exo pathway | Endo pathway | |||||
| Transition State | ||||||
| Products | ||||||
Is this a normal or inverse demand DA reaction?
Diels Alder reaction is a concerted [4+2] pericyclic reaction between a conjugated diene species and a substituted dienophile species. There are 2 types of Diels Alder reactions, depending on the relative energy of the FMO involved for the formation of 2 new sigma bonds of the diene and dienophile species. The reaction has a normal electron demand when it occurs between an electron rich diene species (high energy HOMO) and an electron deficient dienophile (low energy LUMO). Conversely, the reaction has an inverse electron demand when it occurs between an electron poor diene species (low energy LUMO) and an electron rich dienophile (high energy HOMO). [2]
The order of TS's MOs symmetry (figure 2 and 3) depict that the reaction has an inverse electron demand. In this case, both of the HOMO (MO 22) and LUMO (MO 23) of cyclohexadiene have lower energy than the HOMO (MO 19) and LUMO (MO 20) of 1,3-dioxole (dienophile).This is due to the presence of oxygen atoms next to the C-C double bond in dienophile with the oxygen as electron donating group. The delocalisation of lone pair at the p orbital of oxygen atoms into the π electron system of the C-C double bond in 1,3-dioxole makes the dienophile more electron rich thus results in a higher energy HOMO and LUMO. Consequently, the diene LUMO-dienophile HOMO energy gap become smaller whereas the diene HOMO-dienophile LUMO energy gap becomes larger. Therefore, the diene LUMO and dienophile HOMO which is closer in energy overlap better and this interaction dominates in an inverse demand DA reaction.
Nf710 (talk) 23:31, 7 November 2017 (UTC)This is an excellent explanation and understanding
Tabulate the energies and determine the reaction barriers and reaction energies (in kJ/mol) at room temperature. Which are the kinetically and thermodynamically favourable products?Look at the HOMO of the TSs. Are there any secondary orbital interactions or sterics that might affect the reaction barrier energy?
| Species | Energy calculated from semi empirical method (PM6) / kJmol-1 | Energy calculated from DFT method B3LYP (6-31G(d)) / kJmol-1 |
|---|---|---|
| Cyclohexadiene | + 306.77 | - 6.12399 X 105 |
| 1,3-Dioxole | - 137.21 | - 7.00967 x 105 |
| Sum of reactant energy | + 169.56 | - 1.313367 x 106 |
| Exo-TS | + 364.58 | - 1.313199 x 106 |
| Exo-Product | + 99.68 | - 1.313431 x 106 |
| Endo-TS | + 362.05 | - 1.313207 x 106 |
| Endo-Product | + 99.22 | - 1.313435 x 106 |
| Method | Semi empirical method (PM6) | DFT method B3LYP (6-31G(d)) | ||
|---|---|---|---|---|
| Exo | Endo | Exo | Endo | |
| Activation barrier (Eact) /kJmol-1 | + 195.02 | + 192.49 | + 167.53 | + 159.76 |
| Reaction energy /kJmol-1 | - 69.88 | - 70.34 | - 63.79 | - 67.39 |
If a reaction required lesser activation energy to achieve its TS, the reaction is more kinetically favoured and vice versa.
If the reaction has greater reaction energy, the reaction is more thermodynamically favoured.
According to the thermochemistry data obtained from the gaussian output log file using the DFT method B3LYP (6-31G(d)) method, the activation energy required to reach endo-TS is lower by 7.77 kJmol-1 and the reaction energy for endo-product is larger by 3.60 kJmol-1. This depicts the endo-product forms faster and it is kinetically and thermodynamically favourable.
| exo-MO | endo-MO | Type of interactions | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
Compared to the exo-TS, endo-TS has lower energy due to the secondary orbital interactions. The secondary orbital interaction occurs significantly in the endo TS and product from the overlap between the non-bonding p orbitals (lone pairs) of oxygen atoms in 1,3-dioxole and the LUMO of cyclohexadiene across the space. Consequently, this interaction stabilises the TS and lowers the activation barrier thus making the endo-product more kinetically favorable than the exo-product. Note that the unfavourable steric interaction appears less significant in both the endo-TS and product and the resulting repulsion arose from steric clash helps to orient the FMOs of the 2 reactants for a better overlap. Overall the steric effect is so minor thus the reaction is deduced to be dominated by electronic factor. Both of the exo and endo-DA reaction pathways are exothermic. It is observed that the reaction energy for endo-DA reaction is higher than the exo-DA reaction. This is because of the stabilising effect from the secondary orbital interactions in the endo-product and this interaction is greater when compared to its transition state. The stabilised endo-product has a lower energy thus results in a more exothermic reaction which makes it more thermodynamically favourable compared to the exo-product. | ||||||
|
|
Nf710 (talk) 23:44, 7 November 2017 (UTC) This is again a really good explanation. You could have possibly shown that there is a small amount of steric slashing in the exo but not much with a diagram. which is against what happens in a typical Diels alder. But you have come to the correct conclusion. Well done.
Additional information
Link for exercise 1: https://wiki.ch.ic.ac.uk/wiki/index.php?title=Llt15_TS
Link for exercise 3: https://wiki.ch.ic.ac.uk/wiki/index.php?title=Llt15_TS_3
| States in reaction | semi-empirical method (PM6) | |
|---|---|---|
| Exo pathway | Endo pathway | |
| Cyclohexadiene | File:CYCLOHEXADIENE LLT15 FREQ PM6 REDO.LOG | |
| 1,3-Dioxole | File:DIOXOLE LLT15 Q2 FREQ.LOG | |
| Transition State | File:EXO Q2 TS PM6 LLT15.LOG | File:ENDO TS Q2 PM6 LLT15.LOG |
| Product | File:EXO Q2 PRODUCT PM6 LLT15 FREQ.LOG | File:ENDO PRODUCT Q2 PM6 LLT15 FREQ.LOG |
| IRC | File:EXO Q2 TS PM6 LLT15 IRC.LOG | File:Endo TS Q2 LLT15 PM6 IRC.LOG |


