Rep:Kh1015TSEx3RD
Exercise 3 Results and Discussion.
Note to Reader/Marker:
The compartmentalization of the Results and Discussion into 5 parts was based on relevant discussion idea and for convenient navigation during the write-up. Also, the Jmol files in this page could display the relative energies of the MOs but they could not visualize the MOs. In response, the visualized HOMO of each TS was attached next to the Jmol files for viewing purposes.
Part 1: Parameters from Reaction Profile.
Figure 4.3 under Methodology section shows the reaction scheme for Exercise 3.
Referring to Table 5.3.2, calculations at PM6 level showed that only the Diels-Alder minor-regio-isomers were non-spontaneous. The Diels-Alder endo-major-regioisomer (referred to as endo product in Table 5.3.1) was calculated to be the kinetic product (based on lowest activation Gibbs-Free Energy equalled to 83.2 kJ mol-1) and an associated rate of reaction of 0.0165 s-1 at 298.15K. The rate constant was calculated using the formula described under Introduction > Part D. The cheletropic product was calculated to be the thermodynamically favourable product (based on the most negative Δ Gibbs-Free Energy equalled to -155 kJ mol-1). This prediction could be verified experimentally by doing a kinetic study and analysis of ratio of Diels-Alder (including the regioisomers) and cheletropic products formed at rtp.
| States | ||||||||||||
| 5,6-dimethylenecyclohexa-1,3-diene | Sulfur Dioxide | Endo TS | Endo Product | Exo TS | Exo Product | TS of Endo-Minor-Regioisomer | Endo-Minor-Regioisomer | TS of Exo-Minor-Regioisomer | Exo-Minor-Regioisomer | Cheletropic TS | Cheletropic Product | |
| Gibbs-Free Energy (kJ mol-1) | 468 | -313 | 238 | 57.0 | 242 | 56.3 | 268 | 172 | 276 | 177 | 260 | -0.00263 |
| Endo-Major-Regioisomer | Exo-Major-Regioisomer | Endo-Minor-Regioisomer | Exo-Minor-Regioisomer | Cheletropic | |
| Activation Gibbs-Free Energy (kJ mol-1) | 83.2 | 87.2 | 113 | 121 | 106 |
| Δ Gibbs-Free Energy (kJ mol-1) | -97.6 | -98.2 | 17.7 | 22.2 | -155 |
| Predicted Rate of Reaction (x10-6 s-1) | 16,500 | 3,280 | 0.010 | 0.004 | 1.67 |
(Nice to see someone investigating the rates of reaction. There are a few more considerations - including which approximations you are using - when attempting to calculate rates. One of them is the shape of the PES, such as the "wideness" of the barrier. However, the thing that happens to break this the most is there is another barrier: the conversion of the endo and exo products, which is lower than all the other barriers! This means that, under kinetic conditions, the endo isn't the major product Tam10 (talk) 12:59, 16 March 2018 (UTC))
Part 2: Comparison of Reaction Profiles on a Standardized Reaction Coordinate.
Figure 5.3.1 shows the comparison of the Diels-Alder and Cheletropic Reaction Profiles on a Standardized Reaction Coordinate at 298.15 K and 1 atm (PM6 level). In the Standardized Reaction Coordinate, the reactants' coordinates are set to -1, those of TS set to 0 and those of products set to 1. This was done to align the all the reaction states independent of the rate on a common scale such that the relative energetics could be compared between possible reaction paths.
 |
(Some suggestions: 1) If you normalise to the common point (reactants) then you can read off the activation and reaction energies more easily. 2) The numeric values of the x axis have no real meaning so these axis labels can be removed. 3) Placing the values of the activation and reaction energies on the chart itself will further improve legibility (especially where the endo- and exo- products overlap Tam10 (talk) 12:59, 16 March 2018 (UTC))
Part 3: Rationalizing the Reaction Profile Parameters.
Xylylene is an unstable compound because it is a system of 8 π electrons (4n anti-aromatic rule). From the IRC of all the reactions examined (excluding Diels-Alder minor-regio-isomers), it was observed that as xylelene reacted with sulfur dioxide, the 6 membered-ring would end up with a stable 6 π electrons system (4π+2 aromatic rule) in the product. This provided a strong thermodynamic driving force for the reaction with sulfur dioxide to proceed.
For all the reactions examined, there was no identifiable secondary orbital contribution from the Gaussview MO visualizations.
Referring to Figure 5.3.2-5.3.5, the calculated steric interactions were similar for Diels-Alder endo-exo products that react at the same regio-position. Because of similar steric interactions and electronic interactions within each set, the products that reacted at the same position would possess similar activation Gibbs-Free Energy (calculated) as shown in Table 5.3.2. However, when comparing between the two sets of Diels-Alder regio-isomers, it could be seen that the activation Gibbs-Free Energy for the minor-regio-isomer set was much higher than the other set ({113, 121} against {83.2, 87.2} where each element of the sets is in the form {endo, exo} and reported in kJ mol-1). For both sets, the TS formation involved severance of π interactions in the xylylene system. In the major-regio-isomer set, the activation Gibbs-Free Energy was reduced because there was a corresponding formation of favourable aromatic interaction in the 6-membered ring.
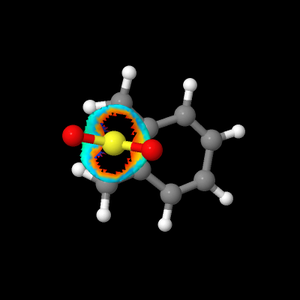
Referring to Figure 5.3.2-5.3.3 and 5.3.6, when comparing between the Diels-Alder major regio-isomer set and the cheletropic product, the steric clash (Red being not favourable and Blue being favourable) in the cheletropic TS was calculated to be higher than those in the Diels-Alder major regio-isomer set. This was due to the approach of two orthogonal lone pairs of S atom towards the 5,6-dimethylenecyclohexa-1,3-diene in the chelotropic TS (Orange colour) that was not present in the Diels-Alder reactions. This contributes to the observation that the calculated activation Gibbs-Free Energy of the cheletropic product, 106 kJ mol-1, was much higher than those of the endo and exo products, 83.2 and 87.2 -1 respectively.
(Nice analysis. It's pretty difficult to get much information about primary interactions with NCI. Perhaps it would be more valuable to get information about the products with NCI Tam10 (talk) 12:59, 16 March 2018 (UTC))
Part 4: IRC.
1. Diels-Alder Products.
1.A. Endo Major-Regio-Isomer.
Referring to Figure 5.3.7, the IRC calculation at PM6 level showed that the C-O and C-S sigma bonds were formed in an asynchronous fashion in a concerted mechanism, with C-O bond forming earlier than C-S bond, and that the TS had been optimized.
 |
 | |||
|
 |
1.B. Exo Major-Regio-Isomer.
Referring to Figure 5.3.11, the IRC calculation at PM6 level showed that the C-O and C-S sigma bonds were formed in an asynchronous fashion in a concerted mechanism, with C-O bond forming earlier than C-S bond, and that the TS had been optimized.
 |
 | |||
|
 |
2.A. Endo Minor-Regio-Isomer.
Referring to Figure 5.3.15, the IRC calculation at PM6 level showed that the C-O and C-S sigma bonds were formed in an asynchronous fashion in a concerted mechanism, with C-O bond forming earlier than C-S bond, and that the TS had been optimized.
 |
 | |||
|
 |
2.B. Exo Minor-Regio-Isomer.
Referring to Figure 5.3.19, the IRC calculation at PM6 level showed that the C-O and C-S sigma bonds were formed in an asynchronous fashion in a concerted mechanism, with C-O bond forming earlier than C-S bond, and that the TS had been optimized.
 |
 | |||
|
 |
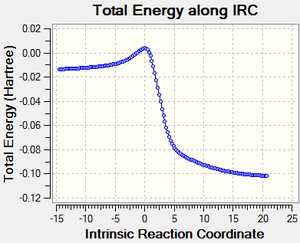
2. Cheletropic Product.
Referring to Figure 5.3.23, the IRC calculation at PM6 level showed that the two C-S sigma bonds were formed in a synchronous fashion in a concerted mechanism and that the TS had been optimized.
 |
 | |||
|
 |
Part 5: Interactive Vibration Animations.
Figures 5.3.27-5.3.31 show the interactive vibration animations.
Figure 5.3.27: Interactive Vibration Animation of the Endo TS (PM6 Method). |
Figure 5.3.28: Interactive Vibration Animation of the Exo TS (PM6 Method). |
Figure 5.3.29: Interactive Vibration Animation of the Endo Minor-Regioisomer TS (PM6 Method). |
Figure 5.3.30: Interactive Vibration Animation of the Exo Minor-Regioisomer TS (PM6 Method). |
Figure 5.3.31: Interactive Vibration Animation of the Cheletropic TS (PM6 Method). |