Rep:Kh1015TSEx2RD
Exercise 2 Results and Discussion.
Note to Reader/Marker:
The compartmentalization of the Results and Discussion into 4 parts was based on relevant discussion idea and for convenient navigation during the write-up.
Part 1: Determining the Type of Diels-Alder Reaction.
Figure 4.2 under Methodology section shows the reaction scheme for Exercise 2.
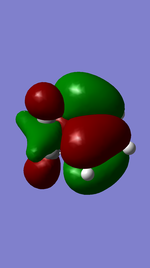
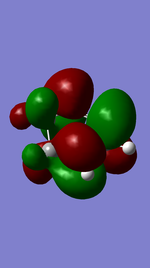
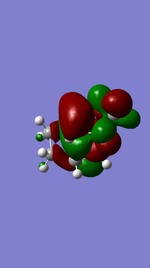
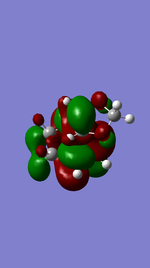
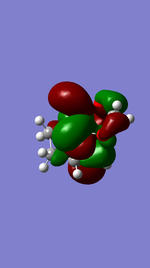
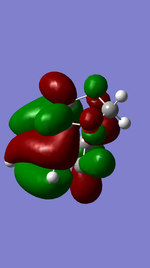
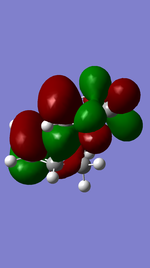
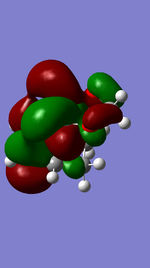
Referring to Figure 5.2.5 and 5.2.10, both of the endo and exo reactions were calculated to be inverse-electron-demand Diels Alder reactions at B3YLP-6-31 G(D) level, whereby the electron-poor dienophile (cyclohexadiene) interacted with the electron-rich diene (1,3-dioxole).
(Fv611 (talk) Very good MO diagrams.)
| TS Symmetry Label (in Increasing Energy Level) | Constituent Fragment Orbital Interactions (Cyclohexadiene - 1,3 Dioxole) |
| Anti-Symmetrical | MO22-MO20 (Bonding) |
| Symmetrical | MO23-MO19 (Bonding) |
| Symmetrical* | MO23-MO19 (Anti-Bonding) |
| Anti-Symmetrical* | MO22-MO20 (Anti-Bonding) |
 |
 |
 |
 |
Endo Product ((3aR,4S,7R,7aS)-3a,4,7,7a-tetrahydro-4,7-ethanobenzo[d][1,3]dioxole).
 | |||
 |
 |
 |
 |
Exo Product ((3aS,4R,7S,7aS)-3a,4,7,7a-tetrahydro-4,7-ethanobenzo[d][1,3]dioxole).
 | |||
 |
 |
 |
 |
Part 2: Parameters from the Reaction Profile.
Referring to Table 5.2.2, it can be seen that the Gibbs-Free Energies of the different species are much larger than the Δ Gibbs-Free Energy in Table 5.2.3. From the calculation of this reaction, it can be observed that the activation Gibbs-Free Energy and energy released/absorbed were only a small fraction relative to the total energy of the system (less than 0.01%).
| States | ||||||
| Cyclohexadiene | 1,3-Dioxole | Endo TS | Endo Product | Exo TS | Exo Product | |
| Gibbs-Free Energy (kJ mol-1) | -612,593 | -701,189 | -1,313,622 | -1,313,849 | -1,313,614 | -1,313,846 |
Referring to Table 5.2.3, calculations showed that both reactions were spontaneous and that the endo product was the kinetically (based on activation Gibbs-Free Energy) and thermodynamically favourable product (based on Δ Gibbs-Free Energy). The endo path had a lower activation Gibbs-Free Energy (160 against 168 kJ mol-1) with higher rate constant at 298.15 K (5.77 against 0.23 x 10-16), and had a more negative Δ Gibbs-Free Energy (-67.4 against -63.8 kJ mol-1), which meant that it was more stable than the exo form. The rate constant was calculated using the formula described under Introduction > Part D. This prediction could be verified experimentally by doing a kinetic study and analysis of ratio of endo to exo products formed at rtp.
| Activation Gibbs-Free Energy (kJ mol-1) | Δ Gibbs-Free Energy (kJ mol-1) | Predicted Rate of Reaction (x10-16 s-1) | |
| Endo-Path | 160 | -67.4 | 5.77 |
| Exo-Path | 168 | -63.8 | 0.23 |
Nf710 (talk) 09:59, 22 March 2018 (UTC) Your energies are correct and you have come to the correct conclusions
Part 3: Secondary Orbital Interactions and Sterics.
Referring to MO 41 for both endo and exo TS in Figure 5.2.15 and 5.2.17, the calculation showed favourable secondary orbital interaction in the endo TS that was not present in the exo TS due to the relative orientation of the two reacting fragments, which would lower the activation energy. Referring to Figure 5.2.16 and 5.2.18, there are significant non-favourable steric interactions (Red being not favourable and Blue being favourable) in the exo TS between the methyl-group and the 6-membered-ring (Green) that was not present in the exo TS due to the relative orientation of the two reacting fragments, which would raise the activation energy. Conversely, both secondary orbital interaction and steric clash accounted for the lower calculated activation energy of the endo path relative to exo path, 160 kJ mol-1 against 168 kJ mol-1.
Endo Product ((3aR,4S,7R,7aS)-3a,4,7,7a-tetrahydro-4,7-ethanobenzo[d][1,3]dioxole).
|
 |
Exo Product ((3aS,4R,7S,7aS)-3a,4,7,7a-tetrahydro-4,7-ethanobenzo[d][1,3]dioxole).
|
 |
Nf710 (talk) 10:01, 22 March 2018 (UTC)This is an excellent way to quantitatively measure the sterics. Well done.
Part 4: Interactive Vibration Animation of TS for Both Endo and Exo Path.
Both Figure 5.2.19 and 5.2.20 contained interactive vibration animation of the Endo and Exo TS (B3YLP-6-31 G(D) level).
Endo Product ((3aR,4S,7R,7aS)-3a,4,7,7a-tetrahydro-4,7-ethanobenzo[d][1,3]dioxole).
Figure 5.2.19: Interactive Vibration Animation of the Endo TS (B3YLP-6-31 G(D) level). |
Exo Product ((3aS,4R,7S,7aS)-3a,4,7,7a-tetrahydro-4,7-ethanobenzo[d][1,3]dioxole).
Figure 5.2.20: Interactive Vibration Animation of the Exo TS (B3YLP-6-31 G(D) level). |
Part 5: Interactive Vibration Animations (PM6 Level).
The IRC calculations at PM6 level showed that the two C-C sigma bonds were formed in a synchronous fashion in a concerted mechanism for both paths and that the TS for both reactions had been optimized.
Endo Product ((3aR,4S,7R,7aS)-3a,4,7,7a-tetrahydro-4,7-ethanobenzo[d][1,3]dioxole).
 |
 |
Exo Product ((3aS,4R,7S,7aS)-3a,4,7,7a-tetrahydro-4,7-ethanobenzo[d][1,3]dioxole).
 |
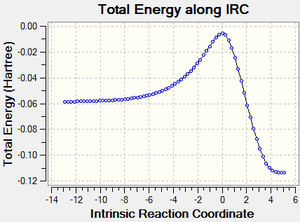 |
Nf710 (talk) 10:05, 22 March 2018 (UTC) This is an excellent section. Your energies are correct and therefore came to the correct conclusions. It was slightly unclear how you investigated the electron demand of the reaction. I see you have put the energies on the MOs diagrams, but you have not said how you got these. Where these the energies on the same PES from a single point energy of the reactants? Or them separate?
