Rep:Jn307Module3:ThisIsIt
The Cope Rearrangement Tutorial
Optimizing the Reactants and Products
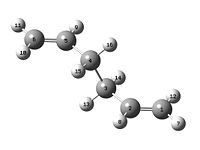
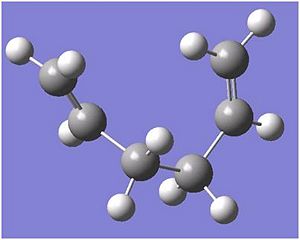
1,5-hexadiene with an "anti" linkage
The molecule was made with the two terminal carbons as far away as possible. The structure was optimised at a HF/3-21G level and the memory was set to 250MB. This file was saved and ran on the Scan in the CollegeDOI:10042/to-3564 . After the molecule has been optimised, the molecle's summary was produced which gives an energy of -231.693 a.u. and the molecule was then symmetrized and then the point group under the button was clicked. This shows that the molecule has a C2 axis. Following the table of data in Appendix 1, the structure was found to corresponds to the anti1 structure.
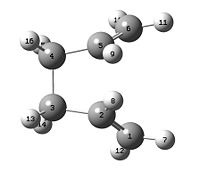
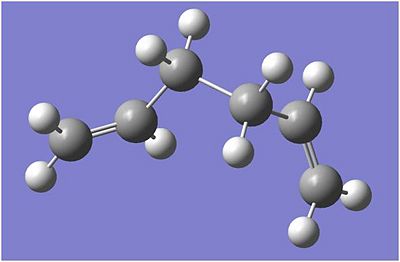
1,5-hexadiene with a "gauche" linkage
Another Structure was then drawn with the terminal carbons close to each other. The structure was again optimised with HF/3-21G level and ran on the Imperial ScanDOI:10042/to-3563 . After the optimization of the gauche structure, the molecule summary was checked in order to find out the total energy which was -231.689 a.u. and the struycture was then symmetrized and the point group was found to be C1, from the Appendix 1, the poitn group and energy confirms that it is indentical to the gauche 6 structure from the appendix.
Comparison of the gauche and the anti structures indicates that the gauche structure is lower in energy by 0.00344182 a.u. and there the gauche was the most stable between the two structures. This may be bacause if the atoms are closer than the molecular orbitals will have better orbital overlap with each other resulting in a lower energy between the atoms.
(c) Lowest Optimised Structure
From the appendix 1, it can be seen the lowest energy conformation of the react molecule is the gauche 3 conformation with an energy of -231.693 a.u. This can be tested by drawing this conformation and optimising it with HF/3-21G, after the optimizationDOI:10042/to-3560 , the energy of the conformation found was -231.663 a.u. which confirms that it is the lowest energy conformation. The atoms are relative closer to each other in order to have favorable interactions between each other however, it is far enough to ensure that there is no steric hinderance on the C=C double bond to raise the energy and hydrogen atoms next to each other.
Comparison of the optimised anti and gauche linkage with the data in appendex one
(e)the Ci anti2 conformation of 1,5-hexadiene.
The above structures does not corresponds to a anti2 conformation so a new structure was drawn in gaussview to resemble the anti2 conformation in Appendix 1. The optimaisation was ran at the HF/3-21G level of theory on the Imperial ScanDOI:10042/to-3565 . After the optimasation, the data was found to have a total energy of -231.693 a.u. and a symmetry point group of Ci which corresponds to the anti2 conformation in the appendix 1.
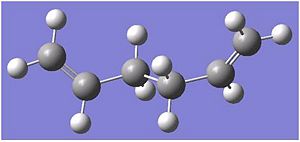
The first optimised structure was then reoptimised at a B3LYP/6-31G again using the Imperial ScanoptDOI:10042/to-3566 10042/to-3566 . The checkpoint file was then opened after the second optimisation and the data was found to have a total energy of -234.55969654 a.u. This energy of the second optimisation was found to be 234.867 a.u. This is a huge significant drop in energy indicating that we are at the minimum or much closer the minimum than after the first optimisation.
The diagrams shows that after the second optimisation, the structure have become straighter and less bent like. the C-C bonds are of a much more sp3 hybidrized structure and is clearly more tetradral in shape. The C=C R groups are also more planer which is what a sp2 hybdrized form should look like and hence there's less steric interaction which would give a lower energy overall.
 |
 |
|---|
Using the optimised B3LYP/6-31G structure, a frequency calculationDOI:10042/to-3567 was then taken using the same level of theory of B3LYP/6-31G. After the frequency analysis has taken place, the vibrations button under the results was used to confirm that all the vibrations are real indicating that the data has minimised. As well as this, it is possible to display the vibrations that the molecule has and to show the dipole displacements vectors. The frequency analysis of the data helps shows that the structure has properly minimised because there is no imagery or negative frequencies shown in the vibrations table.

| Which Energies | Energies/a.u |
|---|---|
| the sum of electronic and zero-point energies | -234.416 |
| the sum of electronic and thermal energies | -234.409 |
| the sum of electronic and thermal enthalpies | -234.408 |
| the sum of electronic and thermal free energies | -234.448 |
Optimizing the "Chair" and "Boat" Transition Structures
Chair Transition stage
(a) Draw an allyl fragment (CH2CHCH2) and optimize it using the HF/3-21G level of theory
The fragment CH2CHCH2 was first drawn in gaussview and optimised using the HF/3-21G level of theory. This was then calculated in Imperial Scan. This is the first fragment and will be used to investigate the transition stage using the TS Berny optimisation and the modredundant level which fixes the molecules distances of the atoms we choose.
TS (Berny)
From the optimised structure of CH2CHCH2 above, the structure was copied and pasted into a new window. A New calculation was then to calculating Opt/Freq and the minimum shown in the optimisation field was changed to TS(Berny). The force constant was chosen to calculated once and opt=noeigen was added to the additional keywords. This is to ensure that only one imaginary frequency is detected during this optimization and can ensure that if more than one imaginary frequency is found than the structure is not good enough. After the calculation has been completed an imaginary frequency of -817.91 cm-1 DOI:10042/to-3558 was found. The frequency of this vibrations shows that as one end of the fragments move closer to each other, the other bond moves away indicating a bond being broken. this corresponds to the cope arrangement which is one bond forming as another one breaks in a concerted manner.
 |
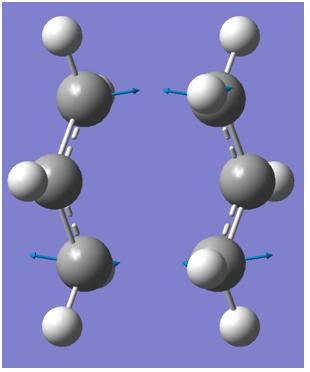 |
Opt=ModRedundant

The molecule was first optimised with the bonds at the terminal ends fixed at 2.2 angstromsDOI:10042/to-3557 . After this optimisation, the bond was allowed to move and a second optimisation was allowed to take place in order to see how the movement of the terminal ends will change to form the cope rearrangement DOI:10042/to-3559 10042/to-3559 . Using the modredundant method with the freezing of the molecular bonds which are forming and breaking, the above image is similar to the benry mechansims. The modredundant mechanism shows a reaction with one bond forming as one of the forming bond is clearly closer than the other one. The Benry structure shows the two forming at equal distances from each other and does no indicate how one will form.
| Energy/a.u. | C-C bond lengths bond breaking | C-C bond lengths bond forming | |
|---|---|---|---|
| Benry optimisation | -231.619 | 2.020 | 2.0197 |
| Mod=redundant method | -231.69166701 | 4.391 | 1.551 |
In the mod=redudant method, the bond forming can easily be distinguish from the bond forming due to the large bond distances between the two, however, in Benry, it can be seen more easily in the vibrations of the structure, the movement of the atoms as the bonds is formed and broken. At the most extreme part of the vibrations, the structure would be indentical to the bond lengths as shown in the mod=redundant method.
Boat Transition Stage
The Boat Transition stage is studied using the second optimised anti2 conformation of the boat structure that was calculated earlier.
QST2 method
This method allows us to distinguish between the reactants and the products of a reactionand the calculation will try to find a transition stage between them. The optimised anti2 conformation was added to a new window and in the same window same window, File → New → Add to MolGroup was selected. This pops us with a screen allow us to show two structures at the same time. I copy and pasted the identical structure into the separate page. The structures were orientated like the picture in the scripts:
| Reactant | Product |
And the hydrogens and carbons in my structures were renumbered to follow the same numbering patterns from the atom list in the edit as well as using the labels for the different atoms. The numbering must be done from 1 and work to number 16 because working randomly would only shift the next highest numbers some place else.
This was then calculated using the Opt+Freq and the TS (QST2), however after this job has been completed, the transition stage does not look like what was expected and is a very distorted shapeDOI:10042/to-3561 as shown below. As it can be seen, the bond on one fragment is trying to bond with the bond on the other end and vice versa, this lead to a very very large steric hindernace between the atoms and electrons should it take place. Gassview would be unable to show the transition stage at this present stage.
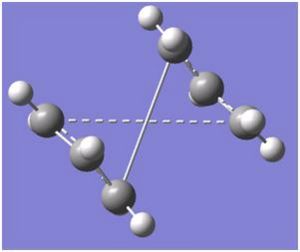
The Structures was then examined once more, the dihedral angle was set to 0 degrees and the angles of the two inner carbons was set to 100 degrees which led to a box like structure as shown below:
| Reactant | Product |
Therefore the set up looks very similar to what the above digrams show:
The image on gaussviewDOI:10042/to-3562 produces a much better transition stage and shows the bonds that is forming and breaking at the same time. The dotted lines clearly shows the electron movement in the transition state. The electrons are planer with respect to the molecule and is easily to see how the transition state can be formed to form the product from the reactants. As well as this, logically, the electrons on terminal carbon easily reacts with the carbon on the other terminal molecule rather than in a distorted manner. The carbons are aligned in the same position which shows a boat transition state.
Anaylsis of the Boat and Chair Transition Stages
Using the IRC method and running it at 50 steps with computing the force constants at every step, it was found that the chair structureDOI:10042/to-3556 needed 46 steps in order to minimise the geometry and reach the transition stage level. The chair structure has an energy of -231.614 a.u. which is lower than the energy of the boatDOI:10042/to-3555 which had a transition of -231.603 a.u., which therefore supports the idea that the chair formation will occur due to a lower transition state and therefore a lower activation energy barrier allow the chair formation to occur first.
 |
 |
|---|
| Conformation | HF/3-21G | B3LYP/6-31G |
|---|---|---|
| Boat | -231.60 | -234.49 |
| Chair | -231.69 | -234.56 |
As the optimisation shows, the energies at the first optimisation of the chair and boat were quite similar with the boat slightly lower in energy which does not make sense as the chair is the more stable product. However, after the second optimisation, the chair conformation is lower in energy and thus more stable. This would indicate that at the first optimisation, the molecules has not reached a minimum equlibrium distance while the second optimisation at a better level of theory shows a much better equalibrium distance.
Diel-Alder Cycloaddition
To find the transition state of this reaction, ethyleneDOI:10042/to-3590 and butadieneDOI:10042/to-3589 was optimised at a AM1 semi-empirical molecular orbital method first using Imperial Scan. After the optimisation a new gaussview window were selected and both optimised reactants were place on the same screen. The ethene were positioned slightly below the butadiene and the freeze co-ordinates method was used. The first optimisationDOI:10042/to-3587 with the bonds frozen was then undertaken with the distance of 2.2 angstroms and then a second optimisationDOI:10042/to-3588 occurs with the bond as a derivative rather than as a fixed bond. The checkfile was then opened in gaussview for further analysis.
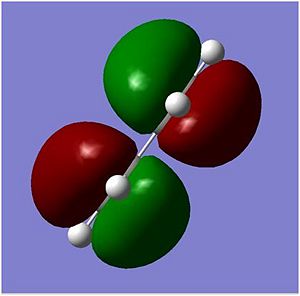
After the second optimization, the cis butudiene molecular orbital diagrams was shown and the results of the HOMO and the LUMO are shown below. The anti-synmmetric HOMO clearly shows two different pi clouds around the two double bonds on the cis butadiene. The symmetric LUMO shows the two clouds in an anti-wavefunction, the orbitals in the middle are drawn together which forms an orbital overlap however, it is next to two anti-wavefunctions which would increases the energy of the molecule and hence even if there's an overlap there is more destabilising energy. The van der Waals radius of the Carbon atom is 170 pm.
 |
 | |
|---|---|---|
| Symmetry | Anti-symmetric | Symmetric |
ethylene+cis butadiene transition structure
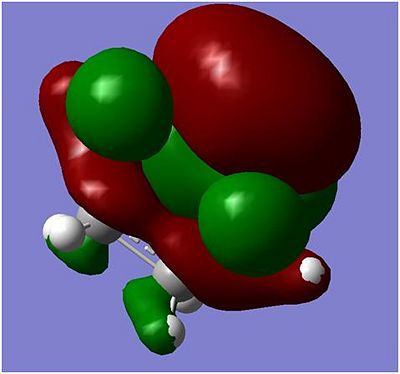 |
 | |
|---|---|---|
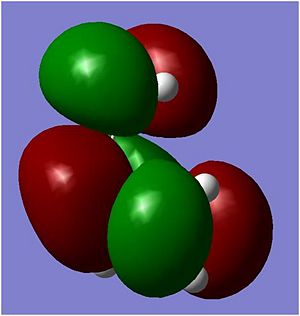
| Symmetry | Symmetric | Anti-Symmetric |
The above diagrams shows that it is the HOMO of the ethene reacting with the LUMO of the cis butadiene which forms the product. This reaction is allowed because the same orbitals can react together in order to minimise energy and therefore a more stable configuration is formed. As well as this, there is sufficient orbital overlap which leads to a more stable product. The 'green' molecular orbitals has overlapped quite nicely with the cis butadiene and the 'red' molecular orbitals are also quite close to each other.
The bonds lengths[1]]] of the C-C is 1.54 angstroms and the C=C bond distance is 1.34 angstroms, the bonds lengths between the forming C-C in this cycloaddition reaction is 2.12 angrstroms. This is bigger than the single and the double bond because it is a transition state and hence the bond between the carbons is not fully formed and hence is only a transition state. The bonds are in the process of forming two new sigma bonds.

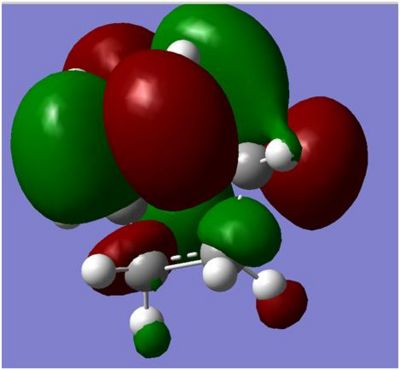
Vibrations of the butadiene
 |
 | |
|---|---|---|
| Frequency number/cm-1 | -955.87 | 147.07 |
The frequency shows the alkene moving towards the cis butadiene in a synchronous manner, this supports the theory that the diels alder reaction occurs in a concerted reaction and both bonds are being formed at the same time via the movement of the electron clouds.
The least positive frequency shows the protons on the alkene just rocking and there's no structral change that can be assessed to the dormation of the two new bonds.
Diels Alder Reaction of Cyclohexa-1,3-diene 1 with maleic anhydride
The QST3 method shown above was first attempted, however, as i was unable to predict the transition stage from this reaction, another way must be taken. From speaking to colleagues, it was found that first using biochem3D and drawing the product of the endo and exo and pressing MM2 would optimised the molecule first abeit in a inaccurate manner. After this optimisation, bonds were drawn in order to show the reactants, the reactants are now in a perfect geometry. The MOPAC was then selected, and the transition stage with RM1 was run in order to get a better geometry of the molecule. This was then saved in a gif file and so it can be imported to gaussview. In gauviess, The calculation was done in TS BENRY, with the force constants calculated once and was allowed to run in Scan. This was done for both the EndoDOI:10042/to-3553 and the ExoDOI:10042/to-3554 transition stage.
By clicking on the summary for the endo and exo transition states, the total energy can be found for both products which is seen below:
Total Energy of the Endo and Exo Conformations Transition State
- Endo = -612.495 a.u.
- Exo = -612.491 a.u.
The energies show that the endo TS is lower in energy and therefore the endo conformation has a lower activation energy and therefore supports the experimental data that the endo conformation is the major product in this reaction.
Bond Lengths through Space
| Bond Lengths/angstroms | Bond Lengths/angstroms | |
|---|---|---|
| Endo | 2.98751 | 2.98752 |
| Exo | 2.78270 | 2.78253 |
As the bond lengths show, the endo's transition state are further away than the exo's transition state. As the exo is closer, it is higher in energy which supports the theory that the exo form is higher in energy as there is more sterics involved than the endo conformation transition state which would be lower in energy.
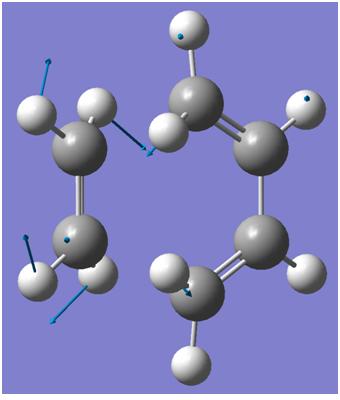
Vibrations
| Endo | Exo | |
|---|---|---|
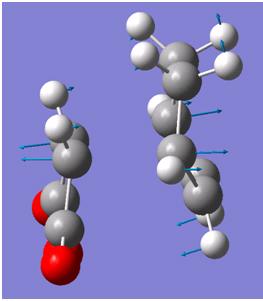 |
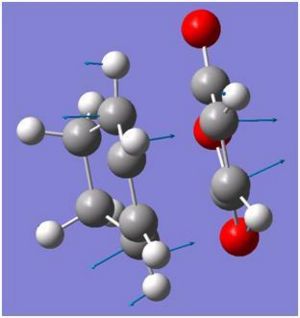 | |
| Frequency/cm-1 | -446.84 | -448.00 |
Both imagery frequencies are similar in the frequency number and both show The double bond on the maleic anhydride moving towards the two double bonds which are also moving towards the maleic anhydride. All the other atoms in both molecules remains stationary as this is occurring.
HOMO and LUMO of the Endo and Exo conformations
| Endo |  |
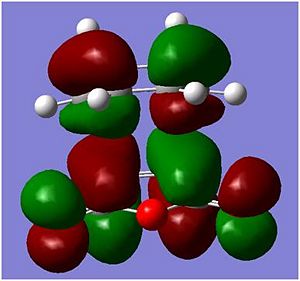 |
|---|---|---|
| Exo | 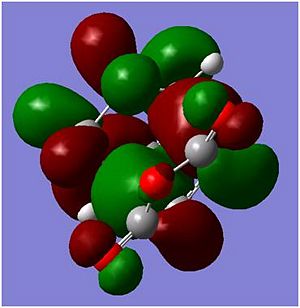 |
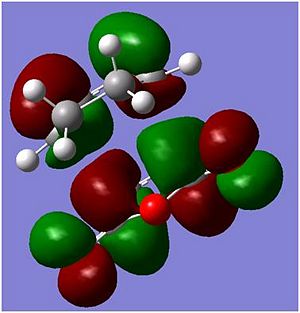 |
In the molecular orbitals diagrams shown above, the -(C=O)-O-(C=O)- fragment on the maleic anhydride on the endo form is closer to the other molecular orbitals, there is secondary orbital overlap[2]]] which would increases the overlap of the incoming diene and the dienophile. This helps support the experimanl data of the endo being the major product in this reaction. However, in the exo form, the -(C=O)-O-(C=O)- fragment on the maleic anhydride is pointing away from all the other atoms in the structure, as it can be seen in the molecular orbital HOMO diagram above, there is almost no interaction between the -(C=O)-O-(C=O)- fragment on the maleic anhydride and the rest of the other orbitals and hence the reaction will not favour the Exo form.
References and Citations
- ↑ ="Chemistry Data Book 2nd Addition in SI units by J. G. stark and H. G. Wallace
- ↑ =" Steric effects vs. secondary orbital overlap in Diels-Alder reactions. MNDO and AM1 studies <DOI:10.1021/jo00384a016 "/