Rep:Jb406p3proj
Module 3 - Physical, part3
Optimising the Chair and Boat conformer transition states of the cope reaction
In this part of the exercise I familiarised myself with the different ways to gain a transition state optimisation by investigating the transition state of the interconversion of hexadiene. This had a clear analogy to Diels-Alder reactions which I would be studying later. It also offererd other advantages: this simple molecule would take less time to calculate, and so the mistakes I would make here would be simple to identify and correct, whilst with more complicated molecules I could be running a non productive calculation for many hours before I realised I had made an error.
First of all I formed the fragment molecules (allyl fragment - CH2CHCH2) and optimised them via a Hartree-Fock optimisation using a 3-21g basis set, to use as the basis of my transition states.
Chair:
BCl3 |
Boat:
BCl3 |
I did this by first constructing the allyl fragments then copy and pasted it so I has 2 in the same file. I then orientated them so that the distance between the carbons forming the bonds was around 2.2Å, and arranged them so that they resembled the transition states.
Part 1: Optimising the transition state via simple gaussian optimisisation
This is more a intermidiatary step, but it gives a good idea to what the transition state will look like. I ran a gaussian optimisation, with calculation of frequency, on my rough transition states, setting the input to optimise to a TS (Berny) and adding in the additional keywords Opt=NoEigen.
The gaussian input file looked like:
# opt=(calcfc,ts,noeigen) freq hf/3-21g geom=connectivity Title Card Required 0 1 C H 1 B1 etc.
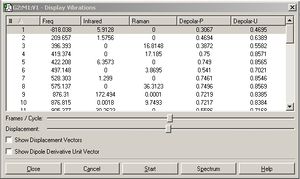
To determine whether the optimisation had occured as planned there should have been a imaginary frequency at -818cm-1. This was not seen after the first optimisation; it required further optimisation of this failed optimisations checkpoint file before I opened the frequency file and saw the imaginary frequency. The vibration of the TS was indeed analogus to the bond breaking and the bond formation seen in the cope rearrangement.
Part 2:Use of the frozen coordinate method
In this part I ensured that the carbons of the seperate allyl fragments that were forming the new bonds were a set distance apart - 2.2Å. This makes the TS more accurate since the bonds are indeed the right distance apart. I did this by going to the Redundant coord editor and selecting the respective atoms - the pairs that were forming and breaking the bonds respectively - and setting the connection to unidentified, and making it so that a fixed distance would always lay between them, the distance being 2.2Å.
The input:
# opt=modredundant rhf/3-21g guess=read geom=connectivity opt to a ts guess force constant (part B) 0 1 C H 1 B1 etc.
The optimised structure:
BCl3 |
This optimised structure is not very different from the original optimisation, but the fragment seperation is more correct.
Part 3:Optimisation by derivision of the bond length
Here I took the checkpoint out file and I changed the nature of the interaction between the bond breaking/forming carbons in the redundant coord editor, to bond, and the changed add to derivative. I then set up a gaussian optimisation, leaving the choice for calculating the force constant as never. I ran the calculation.
Input file:
# opt=(ts,modredundant,noeigen) rhf/3-21g geom=connectivity opt to a ts guess force constant (part d) 0 1 C H 1 B1 etc.
Result:
BCl3 |
Comparison of the seperate parts
When I compared the 3 different optimisations, there did not seem to be any great difference between them just from examination of the molecules within gaussview. however when I used the inquirer tool I found several differences
