Rep:JHT114 Y3C TransitionStates ex3
Exercise 3: Diels-Alder vs Cheletropic
Introduction
The kinetics and thermodynamics of the exo and endo Diels-Alder reactions between SO2 and o-Xylylene were investigated at the PM6 level. In addition, the possible cheletropic reaction was also explored, and the energy profiles of the 3 reactions were compared. In this exercise, the structure of the products were first optimised. These structure were then used to determine the approximate structure of the transition state by breaking the bonds that were formed, then freezing the bond lengths of C-S to approximately 2.4 Å, and C-O to approximately 2.0 Å, and optimised to a minimum. Once the approximate structures were determined, with one imaginary frequency that corresponds to the reaction path, these structures were then optimised to the transition states.

Optimisation of Structures (PM6 Level)
| Table 1: Optimised Reactants at PM6 Level | |||||
|---|---|---|---|---|---|
| o-Xylylene | SO2 | ||||
| Table 2: Optimised Transition States at PM6 Level | ||||||||
|---|---|---|---|---|---|---|---|---|
| Endo | Exo | Cheletropic | ||||||
| Table 3: Optimised Products at PM6 Level | ||||||||
|---|---|---|---|---|---|---|---|---|
| Endo | Exo | Cheletropic | ||||||
Visualisation of Reaction Coordinate
| Endo | Exo | Cheletropic | |
|---|---|---|---|
| Reaction Coordinate | 
|

|
|
| IRC Calculation | 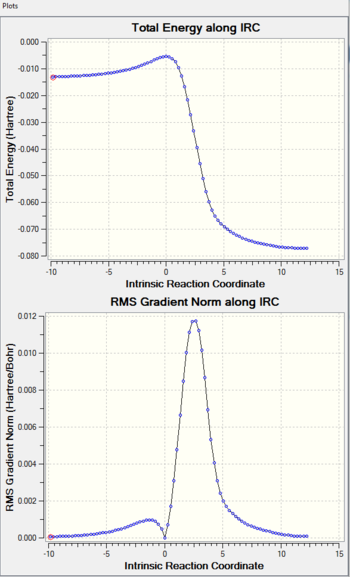
|
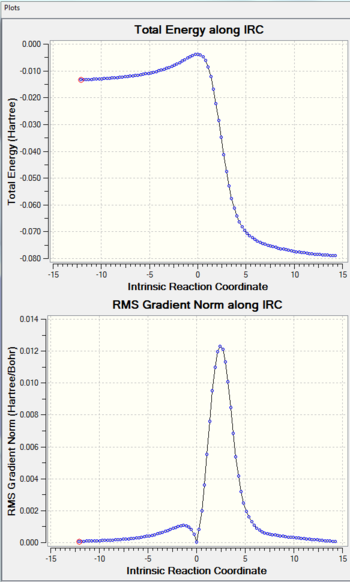
|
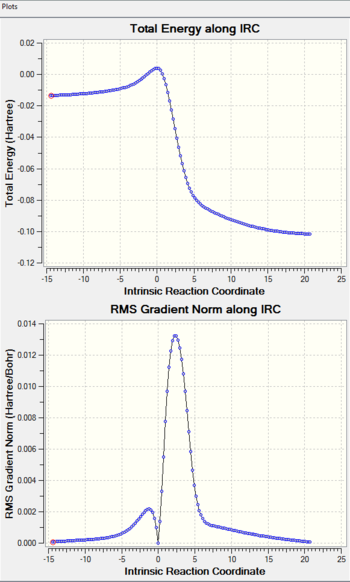
|
| IRC Log Files | File:JHT HDA TS ENDO IRC.LOG | File:JHT HDA TS EXO IRC.LOG | File:JHT CHEL IRC.LOG |
The hetero-Diels-Alder reactions can be seen to be asynchronous, with the C-O bond being formed before the C-S bond. This could possibly be due to the better overlap between the 2p(C)-2p(O) in comparison to the 2p(C)-3p(S), since 2p orbitals would be closer in energy and interact more strongly.
Comparison of Kinetics and Thermodynamics of the Different Routes
| Table 4: Thermochemistry Data at PM6 Level | |
| Species | Sum of electronic and thermal free energies / kJ mol-1 |
|---|---|
| o-Xylylene | 468.783 |
| SO2 | -311.421 |
| Endo TS | 237.762 |
| Exo TS | 241.761 |
| Chel TS | 260.084 |
| Endo Product | 56.983 |
| Exo Product | 56.330 |
| Chel Product | 0.013127 |
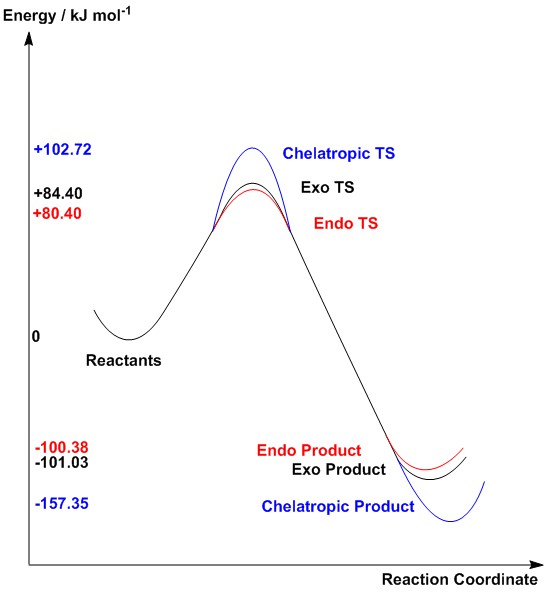
| Table 5: Reaction energy and activation barrier | |||
| Reaction type | Reaction Energy / kJ mol-1 | Activation Barrier / kJ mol-1 | |
|---|---|---|---|
| Diels Alder | Endo | -100.38 | 80.40 |
| Exo | -101.03 | 84.40 | |
| Chelatropic | -157.35 | 102.72 | |
(Please avoid these curved lines in reaction profiles. It's sufficient and tidier to use straight lines between geometries Tam10 (talk) 15:51, 19 February 2018 (UTC))
From the energy profile diagram, a reaction under kinetic control will yield the endo product as the major product, with the exo product as minor product. However, due to the small difference in energy barrier, the ratio of both products are not expected to differ much.
Under thermodynamic control, the chelatropic product will be produced due to the large difference in reaction enthalpy, where there is an appreciable 50 kJ mol-1 difference in reaction free energy
Bonding in 6-membered ring of Xylylene during reaction progress
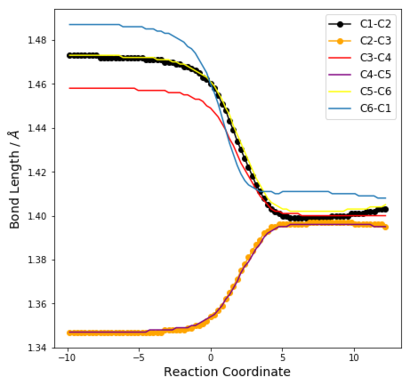
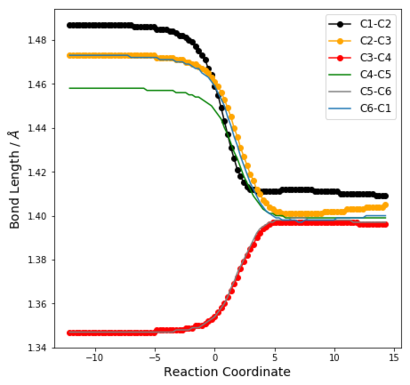
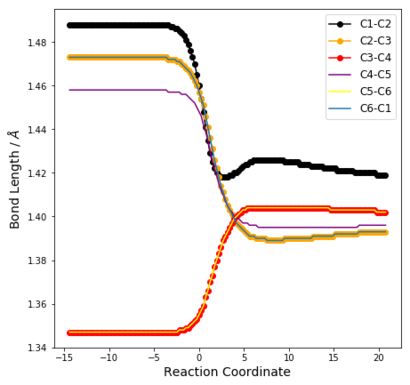
| Table 6: Change in C-C bond distances as reaction progresses | ||
| Endo | Exo | Cheletropic |
|---|---|---|
|
|
|
From Table 6, it can be seen that before the reaction, the bonding in the 6-membered ring resembles that of 2 C=C double bonds (approx 1.345 Å) and 4 sp2-sp2 C-C single bonds [(1.47 ± 0.01) Å]. Thus, there is no resonance stabilisation even though there are 6π electrons in the ring. This is because for xylylene to be aromatic, it would have to exist as a diradical at the methylene groups, which is highly reactive.
In all reaction pathways, a benzene ring is formed, which can be seen from the IRC animations. This is further confirmed by the changes in bond lengths of the 3 structures as illustrated in Table 6, which become significantly shorted than C-C and longer than C=C. Specifically, these distances have values between 1.38 Å and 1.42 Å, which shows the formation of partial double bonds. (c.f. 1.397 Å in benzene and 1.421 Å in graphite)
This explains the high reactivity and of Xylylene at the exo-cis-butadiene fragment, since a chelatropic or hDA reaction results in a resonance energy gain of about 151 kJ mol-1 from the formation of the benzene ring. [1]
(Good analysis. This is the thermodynamic driving force of the reaction Tam10 (talk) 15:51, 19 February 2018 (UTC))
Reaction at Second cis-butadiene fragment of o-Xylylene
Optimised Structures
| Table AAA: Optimised Structures at PM6 Level | ||||||
|---|---|---|---|---|---|---|
| Endo | Exo | |||||
| Transition States | ||||||
| Products | ||||||
IRC Analysis
| Endo | Exo | |
|---|---|---|
| Reaction Coordinate | 
|
|
| IRC Calculation | 
|
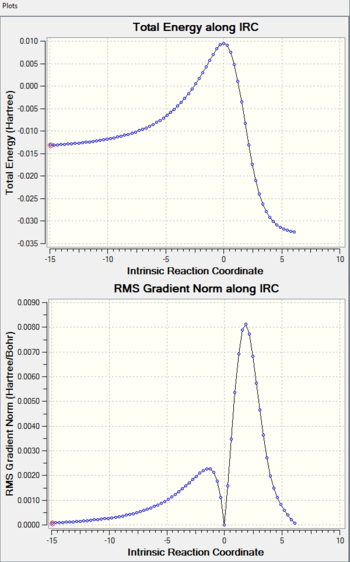
|
| IRC Log Files | File:JHT 2ND ENDO IRC EX3.LOG | File:JHT 2ND EXO IRC.LOG |
Thermodynamic and Kinetic Analysis
| Table BB: Thermochemistry Data at PM6 Level | |
| Species | Sum of electronic and thermal free energies / kJ mol-1 |
|---|---|
| o-Xylylene | 468.783 |
| SO2 | -311.421 |
| 2nd Endo TS | 267.982 |
| 2nd Exo TS | 275.822 |
| 2nd Endo Product | 172.259 |
| 2nd Exo Product | 176.707 |
| Table BB: Reaction energy and activation barrier | |||
| Reaction type | Reaction Energy / kJ mol-1 | Activation Barrier / kJ mol-1 | |
|---|---|---|---|
| Diels Alder | 2nd Endo | 14.90 | 110.62 |
| 2nd Exo | 19.35 | 118.46 | |
The reaction is both kinetically and thermodynamically more unfavourable compared to that at the exo-cis-butadiene fragment. Both the higher activation barrier (by approx. 30 kj mol-1) and reaction energy (by approx. 140 kj mol-1) could be a result of the loss of resonance stabilisation in both the transition state and products.
In fact, the thermodynamic unfavourability is similar to the difference between resonance energy (from formation of benzene) and conjugation energy (from exo-cis-butadiene fragment that remains).
Resonance Energy = 151 kj mol-1 Conjugation Energy = 15 kj mol-1 [2] Difference = 136 kj mol-1 (c.f. 140 kj mol-1 difference between reaction energies)
Thus, o-Xylylene reacts exclusively at the exo-cis-butadiene fragment, and not at the cis-butadiene fragment in the 6-membered ring.
Conclusions
3 different Diels-Alder reactions were explored using computational methods.
The normal electron-demand reaction between the two simplest components of a of a [4+2] cycloaddition, ethene and 1,3-butadiene was investigated at the PM6 level. The reaction was determined to proceed via a thermal supra-supra pathway, in accordance with the Woodward-Hoffmann rules. This can be rationalised from the overlap integrals of the molecular orbitals in concern, where orbitals of the same symmetry interact to give non-zero integrals. The change in C-C bond lengths were also explored, where the two new C-C single bonds were determined to form in a synchronous fashion.
The inverse electron-demand reaction between 1,3-dioxole and 1,3-cyclohexadiene was explored using the B3LYP method with the 6-31G(d) basis set. The endo pathway was found to be both kinetically and thermodynamically favoured due to secondary orbital overlap and steric interactions.
Also, a hetero-Diels-Alder and cheletropic reaction between xylylene and sulfur dioxide was investigated at the PM6 level. The reaction occurs exclusively at the exo-cis-butadiene fragment due to the formation of a resonance-stabilised benzene ring. Kinetically, the endo hDA reaction is most preferred; whereas under thermodynamic conditions, the cheletropic reaction would dominate. The reaction could also be observed to be asynchronous, with the C-O bond being formed first.
Further Work and Exercises 1,2
Further Work: https://wiki.ch.ic.ac.uk/wiki/index.php?title=JHT114_Y3C_TransitionStates_fw
Links to previous exercises:
Exercise 1: https://wiki.ch.ic.ac.uk/wiki/index.php?title=JHT114_Y3C_TransitionStates
Exercise 2: https://wiki.ch.ic.ac.uk/wiki/index.php?title=JHT114_Y3C_TransitionStates_ex2
References
- ↑ V. L. Kolesnichenko, J. Chem. Educ., 2015, 92, 2170–2172. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4795981/#R2
- ↑ D. W. Rogers, N. Matsunaga, and A. A. Zavitsas. J. Org. Chem., 2006, 71(6), pp 2214–2219. https://pubs.acs.org/doi/pdf/10.1021/jo051358m