Rep:JHT114 Y3C TransitionStates ex2
Exercise 2: Reaction of Cyclohexadiene and 1,3-Dioxole
Introduction

The 2 possible pathways for the inverse electron-demand Diels-Alder reaction was investigated using B3LYP method with the 6-31G(d) basis set. The structures were first optimised at the PM6 level (using the same methods as exercise 1) to improve the efficiency of the optimisation process.
The type (inverse/normal Diels-Alder) was determined from the relative energy levels of the diene and the dienophile from single point energy calculations of the first structure of the IRC pathway. The kinetics and thermodynamics of the two possible pathways were also compared.
Optimised Structures
| Table 1: Optimised Strcutures of Reactants at B3LYP/6-31G(d) Level | |||||
|---|---|---|---|---|---|
| Cyclohexadiene | 1,3-Dioxole | ||||
| Table 2: Optimised Strcutures of Transition States at B3LYP/6-31G(d) Level | |||||
|---|---|---|---|---|---|
| Exo TS | Endo TS | ||||
| Table 3: Optimised Strcutures of Products at B3LYP/6-31G(d) Level | |||||
|---|---|---|---|---|---|
| Exo Product | Endo Product | ||||
Molecular Orbital Diagram and Analysis
(Fv611 (talk) Excellent work in this section, both in the MO diagram and in the discussion of electron demand. Well done!)
Exo Pathway
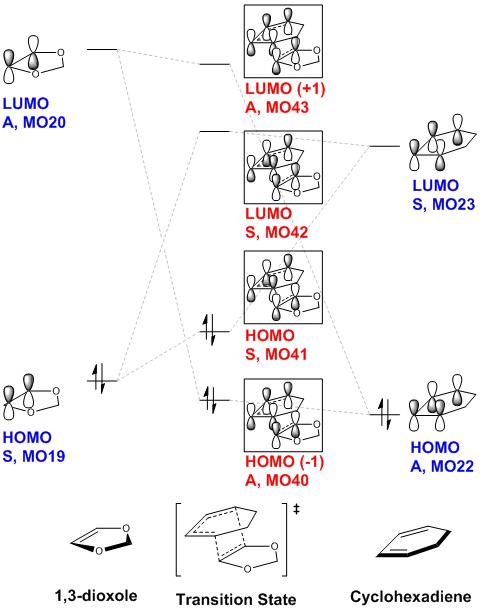
| 1,3-dioxole | Molecular Orbital Diagram | 1,3-cyclohexadiene | ||||
|---|---|---|---|---|---|---|
|
||||||
| LUMO, A, MO20 | LUMO, S, MO23 | |||||
| HOMO, S, MO 19 | HOMO, A, MO 22 |
| Transition State | Frontier Orbitals | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TS HOMO(-1), A, MO40 | TS HOMO, S, MO41 | TS LUMO, S, MO42 | TS LUMO(+1), A, MO43 | ||||||||
The relative energy levels of the reactants are determined from the single point energy calculation of the first structure of the IRC path (corresponding to the reactants). The relative energy levels of the reactants were not compared between separate 1,3-dioxole and 1,3-cyclohexadiene files since the potential energy surface for the two files would be different, resulting in an erroneous comparison.
As in exercise 1, only molecular orbitals with the same symmetry interact with each other to give TS MOs of the same symmetry. The reaction is also thermally controlled and governed by Woodward-Hoffmann rules, which involves the supra-supra interaction between 1,3-cyclohexadiene and 1,3-dioxole. As can be seen from the Transition State molecular orbitals, there is no secondary orbital interaction between the 2p of O and that of cyclohexadiene.
The combination of molecular orbitals giving rise to the Transition State molecular orbitals are:
TS HOMO(-1), A, MO40 = 1,3-dioxole LUMO, A, MO20 + cyclohexadiene HOMO, A, MO22 TS HOMO, S, MO41 = 1,3-dioxole HOMO, S, MO19 + cyclohexadiene LUMO, S, MO23
TS LUMO, S, MO42 = 1,3-dioxole HOMO, S, MO19 - cyclohexadiene LUMO, S, MO23 TS LUMO(+1), A, MO43 = 1,3-dioxole LUMO, A, MO20 - cyclohexadiene HOMO, A, MO22
Nf710 (talk) 23:49, 22 February 2018 (UTC) You say that you have quantitatively compared these results but i do not see it anywhere? It would have been nice if youn coudl have plotted the actual energies or actually shown the MOS for the single point energy.
Endo Pathway
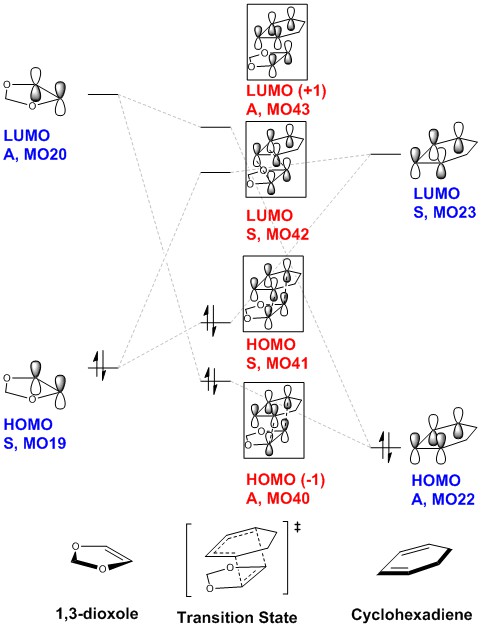
| 1,3-dioxole | Molecular Orbital Diagram | 1,3-cyclohexadiene | ||||
|---|---|---|---|---|---|---|
|
||||||
| LUMO, A, MO20 | LUMO, S, MO23 | |||||
| HOMO, S, MO 19 | HOMO, A, MO 22 |
| Transition State | Frontier Orbitals | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TS HOMO(-1), A, MO40 | TS HOMO, S, MO41 | TS LUMO, S, MO42 | TS LUMO(+1), A, MO43 | ||||||||
Similar to the exo pathway, only molecular orbitals of the same symmetry interact. The difference is that there is now secondary orbital interactions with the 2p orbitals of oxygen, as seen in TS HOMO, S, MO41 . The effect of this is analysed in the sections below.
The combination of molecular orbitals giving rise to the Transition State molecular orbitals are the same as that as the exo pathway:
TS HOMO(-1), A, MO40 = 1,3-dioxole LUMO, A, MO20 + cyclohexadiene HOMO, A, MO22 TS HOMO, S, MO41 = 1,3-dioxole HOMO, S, MO19 + cyclohexadiene LUMO, S, MO23
TS LUMO, S, MO42 = 1,3-dioxole HOMO, S, MO19 - cyclohexadiene LUMO, S, MO23 TS LUMO(+1), A, MO43 = 1,3-dioxole LUMO, A, MO20 - cyclohexadiene HOMO, A, MO22
Normal vs Inverse Electron-demand
The electron-demand of a Diels-Alder reaction can be rationalised from the Frontier Orbitals that are involved in the reaction. A normal Diels-Alder reaction would involve the interaction between the HOMO of the diene the LUMO of the dienophile, as shown in Figure 1 below. This means that the dienophile would be electron-deficient (Low energy LUMO), or that the diene is electron-rich (high energy HOMO), or both. In an inverse electron-demand Diels-Alder reaction, the dienophile is the electron-rich species (High energy HOMO). [1]

From the MO diagram, the reaction investigated can be described as an inverse electron-demand Diels-Alder reaction, since the HOMO of the dienophile (MO 19 ) is higher in energy than the HOMO of the diene (MO 22 ). The dominant interaction would be between the MOs that are closer in energy, which is the HOMO of the 1,3-dioxole and LUMO of 1,3-cyclohexadiene. This behaviour can be further rationalised from the fact that the previously non-bonding 2p orbitals of O can interact in an anti-bonding fashion with the π bond of ethylene fragment of 1,3-dioxole (HOMO, S, MO 19 ), thus increasing its energy relative to a normal π bond. Consequently, an electron-rich and high-energy HOMO is produced on the dienophile, resulting in an inverse electron-demand Diels-Alder reaction.
Nf710 (talk) 23:51, 22 February 2018 (UTC) Its not from the MO diagram, its from actually calculations which you have not shown. You arnt backing up your argument.
Kinetic and Thermodynamic Analysis
| Table 4: Thermochemistry Data at B3LYP/6-31G(d) level | |
| Species | Sum of electronic and thermal free energies / 105 kJ mol-1 |
|---|---|
| 1,3-dioxole | -7.0118878 |
| Cyclohexadiene | -6.1259319 |
| Endo TS | -13.1362215 |
| Exo TS | -13.1361432 |
| Endo Product | -13.1384937 |
| Exo Product | -13.1384578 |
| Table 5: Reaction energy and activation barrier | ||
| Reaction type | Reaction Energy / kJ mol-1 | Activation Barrier / kJ mol-1 |
|---|---|---|
| Endo | -67.4 | 159.8 |
| Exo | -63.8 | 167.7 |
Due to the smaller activation barrier and more exothermic reaction energy, the Endo pathway is both kinetically and thermodynamically favoured. This is in contrast to the typical Diels-Alder reactions, where the exo pathway would be thermodynamically favoured. Nonetheless, this anomaly but could be explained via secondary orbital overlap and sterics.
Secondary orbital interactions and sterics
Transition State
| Reaction type | TS HOMO Diagram | Discussion | ||
|---|---|---|---|---|
| Endo | Secondary orbital overlap present between C15, C17, O19 and O20. The initially non-bonding 2p orbitals on O interact in a bonding fashion with those on C15 and C17, which lowers the activation barrier for the endo pathway.
There is possible some flagpole-like steric clash between H7-H12 and H5-H10, which could destabilise the transition state, but these are outweighed by secondary orbital overlap, resulting in a lower energy TS compared to the exo pathway. | |||
| Exo | Secondary orbital overlap is not present, but there is less steric clash, since there are no H atoms pointing directly towards each other as in the case of the endo TS. |
Product
| Reaction type | TS HOMO Diagram | Discussion | ||
|---|---|---|---|---|
| Endo | Similar to the transition state, secondary orbital overlap is present between C15, C17, O19 and O20. The flagpole-like interactions in the transition state has changed to less destabilising diaxial-like interactions. | |||
| Exo | Secondary orbital overlap not present. However, there is now a small amount flagpole-like steric clash between H14 ,17 and 22. (But not very destabilising since the H22 points into the space between H17 and H14, not directly at H17 or H14). As a result of both the lack of secondary orbital overlap and more steric clashes, the exo-product is less stable compared to the endo product. |
Whilst the kinetic analysis is similar to that of a typical exo vs endo Diels-Alder reaction, the thermodynamic result is different. A normal exo product would be favoured on the basis of less steric clashes, but this is not the case due to the reactants employed in this reaction.
Nf710 (talk) 23:54, 22 February 2018 (UTC) This was a very good section, your energies are correct and you have backed up your arguments with lots of data and jmols. Apart from the single point energy. It it would have been nice if you could have spoke abit about the thermodynamics and kenetics from a theoretical perspective.
Exercise 3, Further Work and Exercise 1
Exercise 3: https://wiki.ch.ic.ac.uk/wiki/index.php?title=JHT114_Y3C_TransitionStates_ex3
Further Work: https://wiki.ch.ic.ac.uk/wiki/index.php?title=JHT114_Y3C_TransitionStates_fw
Link to previous exercise:
Exercise 1: https://wiki.ch.ic.ac.uk/wiki/index.php?title=JHT114_Y3C_TransitionStates
References
- ↑ Z. M. Png, H. Zeng, Q. Ye and J. Xu, Chem. - An Asian J., 2017, 12, 2142–2159. http://onlinelibrary.wiley.com/doi/10.1002/asia.201700442/epdf
