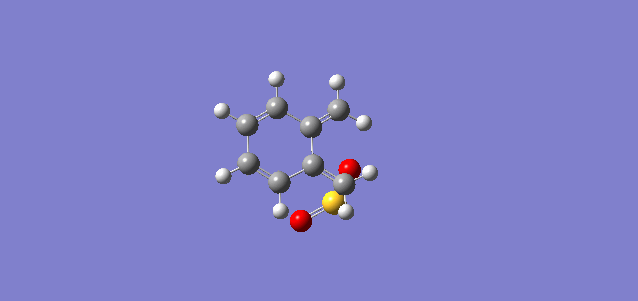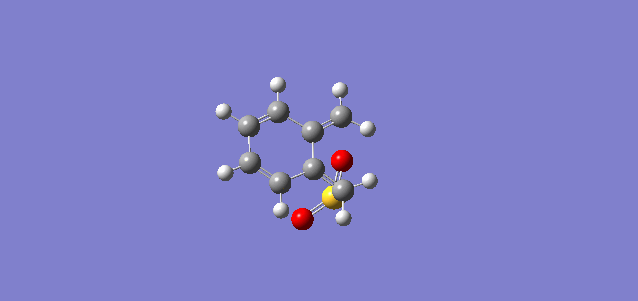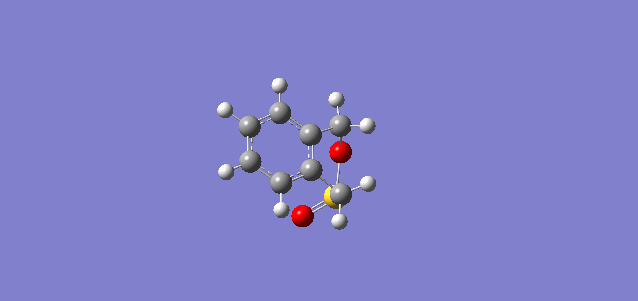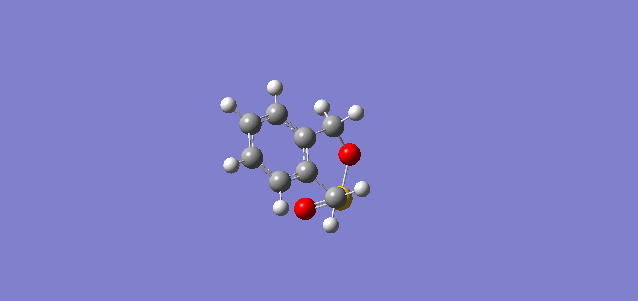Rep:Gcw114: Transition States and Reactivity
Introduction
Transition state

For a chemical reaction, the energy profile diagram can be drawn in Figure 1 to show the reaction coordinate as the reactant is transformed into product. The product is more stable than the reactant. However, in order to form the product, the reactant has to overcome a barrier to the reaction which is the activation energy (EAct). The highest point of this barrier must correspond to some structure which is known as the transition state. The transition state is the highest energy structure with partially formed or broken bond. Transition state cannot be isolated and it is very unstable. Any small change in displacement will result in the formation of the product.
Potential Energy Surface
Using the concept of potential energy surface, we can describe the geometry optimization and transition state in computational and mathematical ways. Each atom would have defined in three coordinates,x,y,and z. Thus, a single atom has 3N coordinates. (N is the number of atoms)After removing the t three rotational and three translational coordinates, the final structure would have 3N-6 coordinates. Due to the complexity in visualizing large dimensional space, we can only normally draw in 3D which at most to be able to picture two of the 3N-6 dimensions which give the PES.
The transition states can be obtained by taking the first and second derivative. In this lab, we will investigate the transition states of the Diel-Alder reaction using GAUSSIAN. We will run a series of optimization of structure to look for transition state and frequency analysis which gives us the second derivative. The Intrinsic Reaction Coordinate (IRC) analysis can ensure that the transition state connects a particular reactant and product. This will give us a better insight into the reaction happened from reactant to product or vice versa.
Nf710 (talk) 11:24, 24 February 2017 (UTC) You should have defined a TS here, but otherwise nice intro explain the degrees of freedom.
Exercise 1: Reaction of Butadiene with Ethene
(Missing a double bond in the product Tam10 (talk) 16:34, 20 February 2017 (UTC))
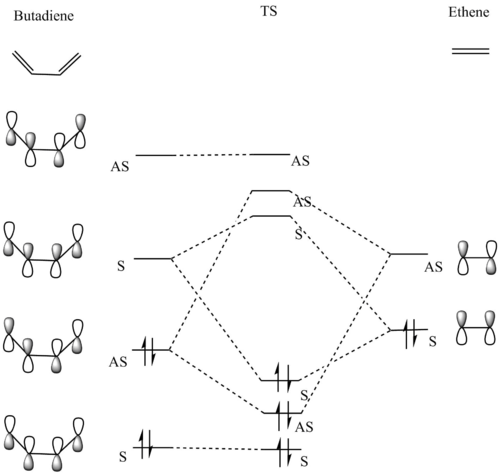
Diagram 2 shows the pushing arrows diagram for the reaction between butadiene and ethene. Both reactants are optimized using semi empirical method with basis set PM 6. The optimised reactant are used to form a TS structure which is later also optimized using the same method. The frontier orbital of reaction is shown in the diagram below.
The Diels-Alder reaction between butadiene and ethene is an inverse demand reaction. This is determined by looking at the position of the transition state of MO symmetry order. The symmetry of HOMO-1, HOMO , LUMO and LUMO-1 are in the order of AS,S,S and AS.
MO Diagram
(Fv611 (talk) 11:49, 24 February 2017 (UTC) Nice MO diagram, but it is missing the drawings of the TS MOs)
Frontier Orbitals of s-cis butadiene and ethene
| Table 1: Frontier Orbitals of s-cis butadiene and ethene | |||||
|---|---|---|---|---|---|
| Species | HOMO | LUMO | |||
|
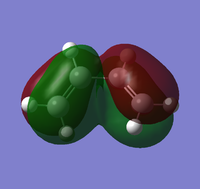
Asymmetric |

Symmetric | |||
|

Symmetric |
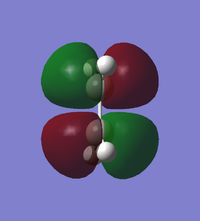
Asymmetric | |||
| Transition state | ||
|---|---|---|
Diagram 4: Transition state of Diels-Alder reaction between butadiene and ethene
Transition state of the reaction of butadiene and ethene are shown in diagram 4. The molecular orbitals formed are displayed and we can clearly see the relation between the frontier orbital and TS symmetry.
(Fv611 (talk) 11:49, 24 February 2017 (UTC) You have the right TS, but the frequency of the vibration is not stated and it has to be started manually from the JMol. Additionally, you don't say whether bond formation is synchronous or asynchronous)
| Table 2: Frontier Orbitals of transition state of reaction s-cis butadiene and ethene | ||||
|---|---|---|---|---|
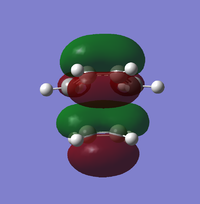
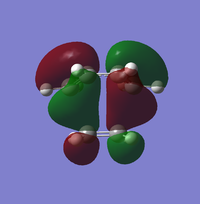
| Molecular Orbital | LUMO +1 | LUMO | HUMO | HUMO-1 |
| Bonding | 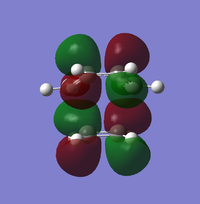
|
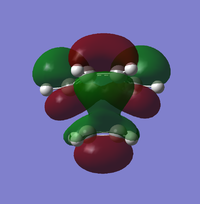
|
|
|
| Symmetry | Asymmetric | Symmetric | Symmetric | Asymmetric |
ALong the reaction coordinate, for reaction to occur, both reactants has to come in the same symmetry.The TS HOMO-1 (bonding) and TS LUMO+1 (antibonding) have resulted from the asymmetrical HOMO of butadiene and asymmetrical LUMO of ethene. On the other hand, the interaction between symmetrical LUMO of butadiene and symmetrical HOMO of ethene has caused the TS HOMO (bonding) and LUMO(antibonding).
The bonding reaction would have a positive integral while the antibonding reaction would have a zero integral. When a symmetrical MO reacts with an asymmetrical MO the overlap integral is zero. Besides that, the stabilising effect of bonding interaction will cancel out the destabilising effect of antibonding interaction.Hence, there are not interaction between symmetrical MO and asymmetrical MO.
For the interaction of symmetrical pair and asymmetrical pair, the overlap integral is non-zero, the bonding one would have a stabilising effect whereas the antibonding will have a destabilising effect.
(Fv611 (talk) 11:49, 24 February 2017 (UTC) It is not clear what do you mean by the bonding and antibonding reaction. Both symmetry-allowed interactions (A/A and S/S) give one bonding and one antibonding MO, with a non-zero overlap integral)
Bond Length Analysis
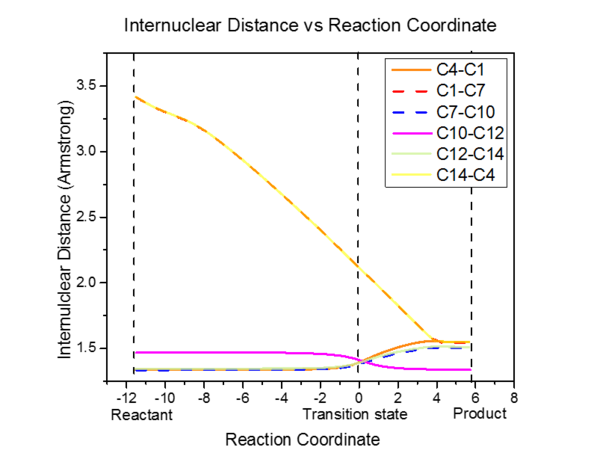
The table below shows the change of length in C-C bonds from reactant to product.
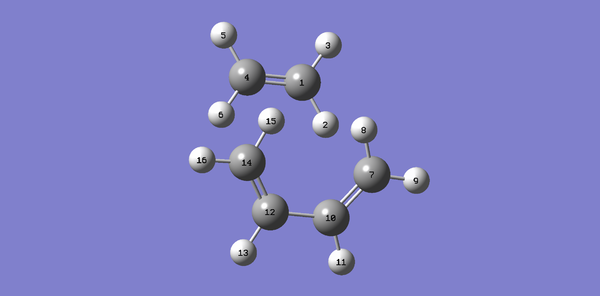
| Table 3: C-C bonds length from reactant to product | |||||||
|---|---|---|---|---|---|---|---|
| Reactant | TS | Product | Literature Values for C-C bond length | ||||
| Bond | Bond length (angstrom) | Bond | Bond length (angstrom) | Bond | Bond length (angstrom) | ||
| C1-C4 | 1.327 | C1-C4 | 1.382 | C1-C4 | 1.541 | sp3C-sp3C | 1.54 |
| C1-C7 | N/A | C1-C7 | 2.114 | C1-C7 | 1.540 | ||
| C7-C10 | 1.335 | C7-C10 | 1.380 | C7-C10 | 1.501 | sp3C-sp2C | 1.50 |
| C10-C12 | 1.468 | C10-C12 | 1.411 | C10-C12 | 1.338 | ||
| C12-C14 | 1.335 | C12-C14 | 1.380 | C12-C14 | 1.501 | sp2C-sp2C | 1.48 |
| C14-C4 | N/A | C14-C4 | 2.115 | C14-C4 | 1.540 | ||
From reactant to product,
1. The C1-C4, C12-C14 and C7-C10 change from double bond to single bond. Hence, the bond is lengthened.
2. The C10-12 changes from a single bond to double bond. Hence, the bond is shorten
3. C1-C7 and C14-4 are the newly formed bonds. These two bonds are with the same length and the internuclear distance reduced.
As for the transition state, the bond length of all bonds is in between their bond length for reactants and products except for C1-C7 and C14-4. The Van Der Waals radius of C-C is 170pm (1.7 angstrom). For C1-C7 and C14-4, the bond length is in between 3.4 angstrom (two carbon bond length) and 1.54 angstrom (literature value for sp3C-sp3C)
Exercise 2: Reaction of Cyclohexadiene and 1,3-Dioxole
Reaction Mechanism:Exo and Endo
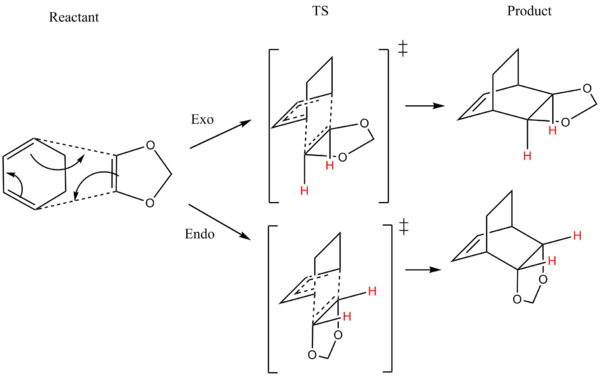
The reaction of cyclohexadiene and 1,3-dioxole can undergo two reaction pathway: Endo an Exo. The 1,3-Dioxole approaches the cyclohexadiene at different orientations to forms two transition states as shown in diagram 7. Both starting reactants cyclohexadiene and 1,3-dioxole are first optimized using semi-empirical method with PM6 basis set then higher DFT method with B3LYP631Gd basis set. The optimized reactants are used to from a proposed structure of TS where it also undergoes the same optimization process as before. The IRC is run to determine the reaction coordinate of the Endo and Exo pathway. The results are discussed in the session below.
(B3LYP is a hybrid DFT functional. 6-31G(d) (aka 6-31G*) is the basis set Tam10 (talk) 16:34, 20 February 2017 (UTC))
MO Diagram
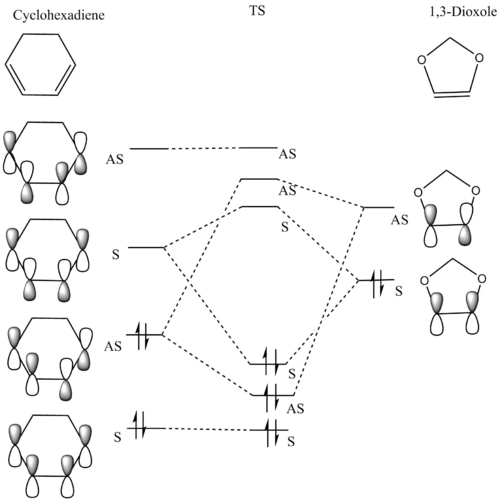
Frontier Orbitals of Cyclohexadiene and 1,3-Dioxole
| Table 4: Frontier Orbitals of Cyclohexadiene and 1,3-Dioxole | |||||
|---|---|---|---|---|---|
| Species | HOMO | LUMO | |||
|
Asymmetric |
Symmetric | |||
|
Symmetric |
Asymmetric | |||
| Table 5: Transition state and product of the Endo and Exo pathway. | ||||||
|---|---|---|---|---|---|---|
| Pathway | Transition State | Product | ||||
| Exo | ||||||
| Endo |
|
|||||
The Diels-Alder reaction between Cyclohexadiene and 1,3-Dioxole is an inverse demand reaction. This is determined by looking at the postion of the transition state of MO symmetry order. The symmetry of HOMO-1, HOMO , LUMO and LUMO-1 are in the order of AS,S,S and AS.
Reaction Energies
| Table 7: Energy data obtained from the reaction of Cyclohexadiene and 1,3-Dioxole. | ||
|---|---|---|
| Temperature/ K | 298.150 Kelvin
Sum of electronic and thermal free Energies (Hartree/Particle) |
0 Kelvin
Sum of electronic and zero-point energies (Hartree/Particle) |
| Endo reactants | 0.076335 | 0.118543 |
| Endo TS | 0.137941 | 0.172488 |
| Endo Product | 0.037807 | 0.070679 |
| Exo reactants | 0.079583 | 0.118829 |
| Exo TS | 0.138903 | 0.173265 |
| Exo Product | 0.037977 | 0.070929 |
| Table 8: Activation Energies and Reaction energies of Cyclohexadiene and 1,3-Dioxole | ||||
|---|---|---|---|---|
| 298.150 Kelvin | O Kelvin | |||
| Reaction Pathway | Activation barrier(kJ/mol) | Reaction energy(kJ/mol) | Activation barrier(kJ/mol) | Reaction energy(kJ/mol) |
| Endo pathway | 160.1756 | -100.1728 | 140.2570 | -124.4464 |
| Exo pathway | 154.2320 | -108.1756 | 141.5336 | -124.5400 |
(We ask for you to calculate reactants as the sum of reactants, instead of a geometry including both xylylene and SO2. The issue is down to consistency - there is a huge PES to explore for the reactants, so it's extremely irreproducible Tam10 (talk) 16:34, 20 February 2017 (UTC)
Nf710 (talk) 11:33, 24 February 2017 (UTC) These should be done at B3LYP it is a much better method.
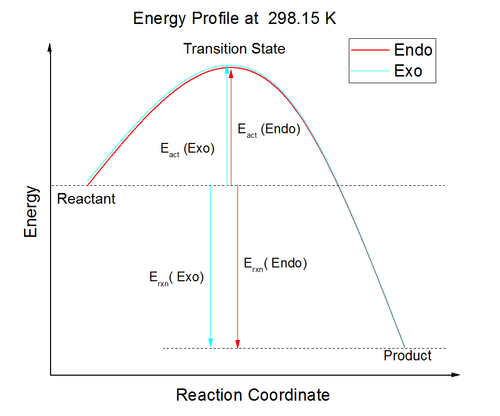
The Endo pathway has slightly lower activation energy barrier which makes the endo product as a kinetically favorable product. The kinetic product forms much quicker than endo product. The Exo product is a thermodynamically favorable product and there is less steric interaction.
From the HOMO of transition states, there is secondary orbital interaction in Endo pathway. The secondary orbital interaction has lowered the activation energy barrier by interacting between non-bonding atoms. From the energy profile, endo has lower activation energy due to the secondary interaction between carbon and oxygen.
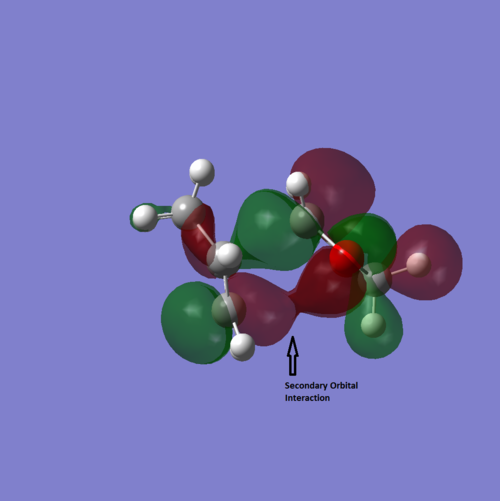
Nf710 (talk) 11:35, 24 February 2017 (UTC) Nice diagram of secondary orbital interaction. It is a shame you calculated the energies with the wrong level of theory as you have got the wrong conclusions for the thermo product.
Exercise 3: Diels-Alder vs Cheletropic
In the exercise, xylylene and sulphur dioxide is react through Diels-Alder or Cheletropic pathway.
Reactant
| Table 9: Structure of xylylene and sulphur dioxide | |||||
|---|---|---|---|---|---|
| Xylylene | Sulphur Dioxide | ||||
Diels-Alder
| Table 10: Transition state and product of Xylylene and sulphur dioxide through Diels-Alder. | |||||||
|---|---|---|---|---|---|---|---|
| Exo | Endo | ||||||
|
| ||||||
|
| ||||||
Cheletropic
| Table 11: Transition state and product of Xylylene and sulphur dioxide through Cheletropic. | |||||
|---|---|---|---|---|---|
| Transition State | Product | ||||
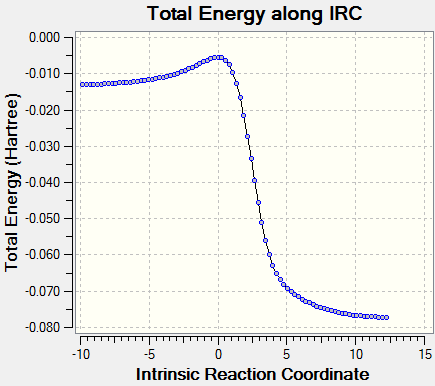
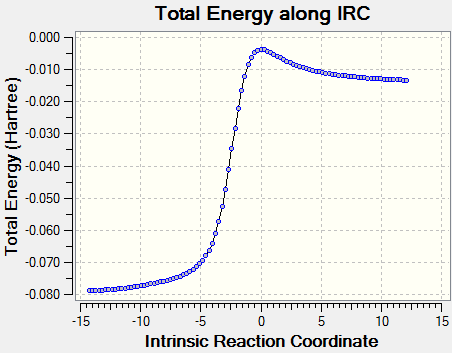
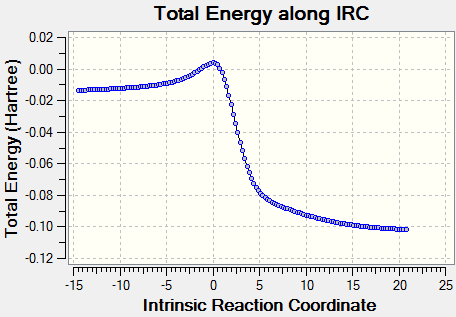
Intrinsic reaction coordinate is used to determine the reaction profile from reactant to product. The successful IRC will shows the reaction profiles as above. Xylylene is unstable. From the IRC, it can be observed that when the 6-membered ring is formed the electrons quickly delocalised.The bond is delocalised and it is more reactive.
Reaction Energies
The data is calculated from semi-empirical PM6 optimised reactant, product, TS from IRC output except exo reactants
| Temperature/ K | 298.150 Kelvin
Sum of electronic and thermal free Energies (Hartree/Particle) |
0 Kelvin
Sum of electronic and zero-point energies (Hartree/Particle) |
|---|---|---|
| Endo reactants | 0.067932 | 0.114802 |
| Endo TS | 0.090561 | 0.126590 |
| Endo Product | 0.021700 | 0.057503 |
| Exo reactants | 0.060496 | 0.116965 |
| Exo TS | 0.092077 | 0.128171 |
| Exo Product | 0.021455 | 0.056645 |
| Cheletropic reactants | 0.070992 | 0.114807 |
| Cheletropic TS | 0.099061 | 0.095059 |
| Cheletropic Product | -0.000002 | 0.034556 |
| 298.150 Kelvin | O Kelvin | |||
|---|---|---|---|---|
| Reaction Pathway | Activation barrier(kJ/mol) | Reaction energy(kJ/mol) | Activation barrier(kJ/mol) | Reaction energy(kJ/mol) |
| Endo pathway | 58.8354 | -120.2032 | 30.6488 | -148.9774 |
| Exo pathway | 82.1106 | -101.5066 | 29.1356 | -156.832 |
| Cheletropic pathway | 72.9794 | -184.5844 | 51.3448 | -208.6526 |
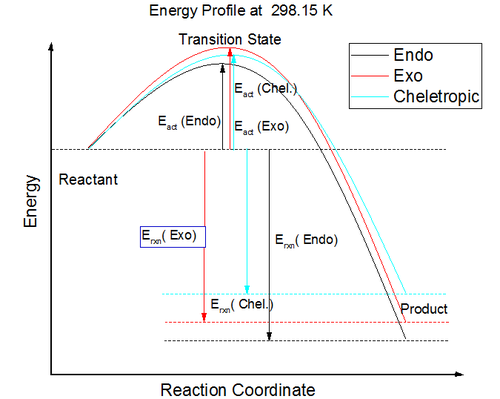
From the energy profile, The Exo product is a thermodynamically favored product, while the endo product is a kinetically favorable product.
Conclusion
Exercise 1: The Diels-Alder reaction between butadiene and ethene is a normal demand reaction. The C-C bond length changes from reactant to product.
Exercise 2: The Diels-Alder reaction between Cyclohexadiene and 1,3-Dioxole is an inverse demand reaction. Endo product is the kinetic product with secondary orbital interaction which lowers the activation energies barrier.
Exercise 3: Xylylene and sulphur dioxide can be reacted through Diels-Alder or Cheletropic pathway. The thermodynamically favored product is exo product. Endo product is kinectically favorable.