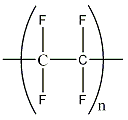
Polytetrafluoroethylene
General
Polytetrafluoroethylene (PTFE) is a fluoropolymer and was serendipitously discovered in 1938 by Roy J. Plunkett. It entered the commercial market in 1946 and is now much better know as Teflon, a brand name given by DuPont.
PTFE has the lowest known coefficiont of friction of any solid material, 0.1 or less. It is also very unreactive. This is why it is used as a non-stick coating for pans, general cookware, chemical containers and pipework. According to DuPont its melting point is 327 °C, but PTFE’s properties degrade above 260°C.
Uses
Some of PTFEs uses are to coat some hardened armourpiercing bullets. this is to reduce the wear on the inside of the firearms, when the bullets are fired.
Due to the fact that it has such a low coefficient of friction it is used a lot where parts slide over each other a lot, e.g. bearings, bushings, gears, slide plates, etc. The only problem here is that PTFE is quite susceptible to wear. Because PTFEs performance is so much better than for example acetal or nylon, mineral oils molybdenum disulfide are integrated into PTFEs structure to overcome the wear problem.
The most well know use for PTFE is for making nonstick pans. But it is also someimes used on carpets and fabrics, to make them extra stain resistant. Another big use for PTFE is in medicine. This is because bodies seldom reject it, so therefore it can be used for making artificial body parts.

Synthesis
The complete synthesis actually takes the following steps:
CaF2 + H2SO4 --> CaSO4 + 2HF
CH4 + 3Cl2 --> CHCl3 + 3HCl
CHCl3 + 2HF --> CHClF2 + 2HCl
2CHClF2 --> CF2CF2 + 2HCl
PTFE is synthesised form the monomer tetrafluoroethylene, using a process known as free radical vinyl polymerization; under pressure and using free radical catalysts:
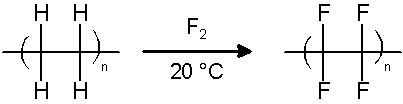
another way for PTFE to be produced is by directly substituting the hydrogen atoms in polyethylene with fluorine. This is done in the gas pahse at 20 °C:
References
http://en.wikipedia.org/wiki/Polytetrafluoroethylene
http://physchem.ox.ac.uk/MSDS/PO/polytetrafluoroethylene.html
http://www.bocedwards.com/pdf/P120-09-025%20PTFE%20.pdf
http://www.pslc.ws/mactest/ptfe.htm
Roberts, Royston M.; Serendipity: Accidental Discoveries in Science; John Wiley and Sons; New York; 1989.
http://www.eng.utah.edu/~nairn/mse/students/MSE3410/Teflon/synthesis.html