Pakwiki
BH3 molecule
Summary
Method and Basis Set: B3LYP/6-31G(d,p)
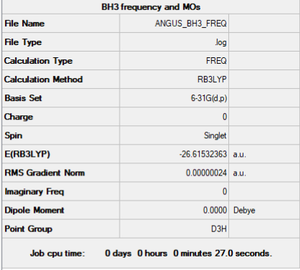
Optimisation
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000002 0.001800 YES RMS Displacement 0.000001 0.001200 YES
Link: File:ANGUS BH3 FREQ.LOG
Frequency Analysis
Low frequencies --- -0.7840 -0.5083 -0.0054 9.7343 14.1292 14.1509 Low frequencies --- 1163.0162 1213.1960 1213.1987
Rotatable 3d image of optimized BH3
Vibrational spectrum for BH3
| wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1163 | 93 | A2 | yes | out-of-plane bend |
| 1213 | 14 | E | very slight | bend |
| 1213 | 14 | E | very slight | bend |
| 2582 | 0 | A1 | no | symmetric stretch |
| 2715 | 126 | E | yes | asymmetric stretch |
| 2715 | 126 | E | yes | asymmetric stretch |
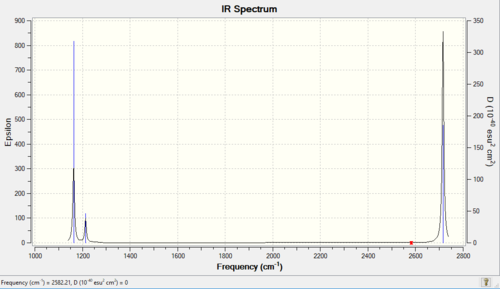
Only 3 bands are observed in the experimental spectrum of BH3. Although there are 6 vibrational frequencies calculated according to GaussView, vibrational modes 2 and 3, 5 and 6 are degenerate as their stretching frequencies are the same. So 4 bands should be observed. However, vibrational modes 4 is highly symmetric and there is no overall dipole moment. As a result, it is not IR active.
Molecular Orbitals of BH3
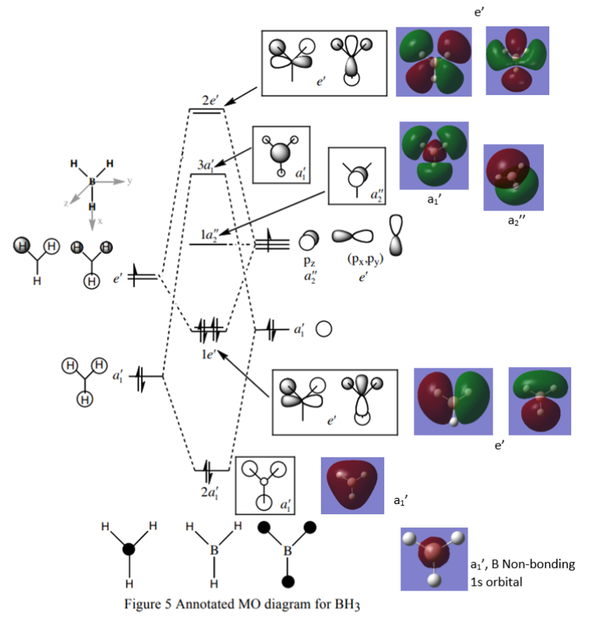
There are only slight differences between the real and LCAO MOs, especially for 3a1 and 2e MOs. Other LCAO MOs are the same as the real MOs, suggesting high accuracy of MO theory. MO theory is useful in predicting the shapes and energies of the MOs, and hence the properties of the molecules.
Well noticed that there are differences between the real and LCAO Mos, to improve you could actually discuss further what the differences actually are. Smf115 (talk) 21:32, 17 May 2019 (BST)
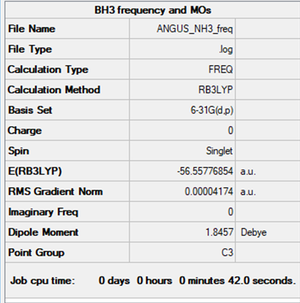
NH3 molecule
Summary
Method and Basis Set: B3LYP/6-31G(d,p)
Optimisation
Item Value Threshold Converged? Maximum Force 0.000100 0.000450 YES RMS Force 0.000042 0.000300 YES Maximum Displacement 0.000344 0.001800 YES RMS Displacement 0.000115 0.001200 YES
Link: File:ANGUS NH3 FREQ.LOG
Frequency Analysis
Low frequencies --- -33.0992 -33.0981 -12.4484 -0.0046 0.0113 0.0466 Low frequencies --- 1088.6659 1694.0140 1694.0140
Rotatable 3d image of optimized NH3
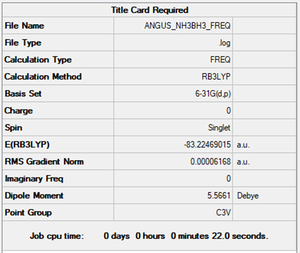
NH3BH3 molecule
Summary
Method and Basis Set: B3LYP/6-31G(d,p)
Optimisation
Item Value Threshold Converged? Maximum Force 0.000157 0.000450 YES RMS Force 0.000062 0.000300 YES Maximum Displacement 0.000554 0.001800 YES RMS Displacement 0.000297 0.001200 YES
Link: File:ANGUS NH3BH3 FREQ.LOG
Frequency Analysis
Low frequencies --- -0.0778 -0.0456 -0.0127 19.1319 19.2987 35.9786 Low frequencies --- 265.2495 633.0064 639.9688
Rotatable 3d image of optimized NH3BH3
Association Energies: Ammonia-Borane
Results and Calculations
From the Summary Tables of NH3, BH3 and NH3BH3, the energy are reported as follows:
Method and Basis Set: B3LYP/6-31G(d,p)
- E(NH3)= -56.55777 a.u.
- E(BH3)= -26.61532 a.u.
- E(NH3BH3)= -83.22469 a.u.
- ΔE=E(NH3BH3)-[E(NH3)+E(BH3)]
ΔE=[-83.22469-(-56.55777-26.61532)]a.u. ΔE=-0.0516 a.u. ΔE=-135 kJ/mol
- The association energy of ammonia borane is -135 kJ/mol.
- The association energy of a C-C bond is about -350 kJ/mol and the association energy of a C-H bond is about -410 kJ/mol.
- The bond strength of B-N dative bond is three times weaker than that of C-C bond. As a conclusion, the B-N dative bond is weak.
Clear presentation of the energies, correct calculation and accuracy of the final reported answer. Your comparisons to evaluate the bond strength are good, you're just missing references for your literature values! Smf115 (talk) 21:31, 17 May 2019 (BST)
NI3 molecule
Summary
Method and Basis Set: RB3LYP/Gen

Optimisation
Optimised N-I distance: 2.18363 Angstrom
Item Value Threshold Converged?
Maximum Force 0.000063 0.000450 YES
RMS Force 0.000038 0.000300 YES
Maximum Displacement 0.000476 0.001800 YES
RMS Displacement 0.000273 0.001200 YES
Link: File:ANGUS NI3 FREQ.LOG.log
Frequency Analysis
Low frequencies --- -12.7347 -12.7286 -6.2858 -0.0040 0.0188 0.0634 Low frequencies --- 101.0320 101.0328 147.4111
Rotatable 3d image of optimized NI3
Project Section: Lewis acids and bases
Conformers and symmetries of Al2Cl4Br2

Clear presentation of the isomers and the correct assignment of the symmetries, you have repeated your C1 isomer though and have missed the one where the Br groups can be terminal of the same Al. Smf115 (talk) 08:02, 22 May 2019 (BST)
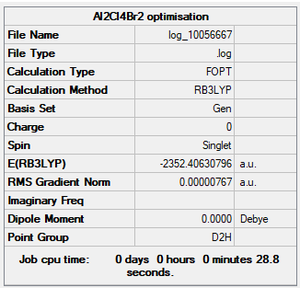
Isomer with 2 bridging Br ions
Summary
Method and Basis Set: RB3LYP/Gen
Optimisation
Item Value Threshold Converged?
Maximum Force 0.000029 0.000450 YES
RMS Force 0.000011 0.000300 YES
Maximum Displacement 0.000230 0.001800 YES
RMS Displacement 0.000101 0.001200 YES
Link: File:Angus cis opt freq.gjf
Frequency Analysis
Low frequencies --- -4.5023 -3.1305 -2.3662 -0.0045 0.0067 0.0106 Low frequencies --- 10.5714 65.1250 88.5355
Rotatable 3d image of isomer with 2 bridging Br ions
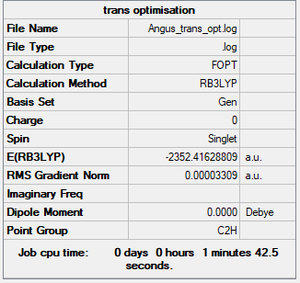
Isomer with trans terminal Br and bridging Cl ions
Summary
Method and Basis Set: RB3LYP/Gen
Optimisation
Item Value Threshold Converged?
Maximum Force 0.000053 0.000450 YES
RMS Force 0.000019 0.000300 YES
Maximum Displacement 0.000444 0.001800 YES
RMS Displacement 0.000170 0.001200 YES
Link: File:Angus trans opt freq.gjf
Frequency Analysis
Low frequencies --- -4.9219 -3.1867 -0.0096 0.0060 0.0064 2.6066 Low frequencies --- 14.9013 52.1840 73.8329
Rotatable 3d image of isomer with trans terminal Br and bridging Cl ions
Good inclusion of all the necessary information about the structures and calculation and the energies imply that you correctly implemented the pseudopotential. However, the summary tables should be from the Frequency calculations and you are missing the corresponding frequency log files. The files submitted are input scripts and the D2h one, in particular, doesn't have the pseudopotential set up correctly. Smf115 (talk) 08:10, 22 May 2019 (BST)
Energy Analysis
Energies of conformers
- Energy of isomer with 2 bridging Br ions: -2352.40631 a.u.
- Energy of isomer with trans terminal Br and bridging Cl ions: -2352.41629 a.u.
- Relative energy of these isomers: 0.00507 a.u. = 26 kJ/mol
Relative stability of the two isomers
- From the energies of the isomers, it can be concluded that the isomer with 2 bridging Br ions is less stable as it has a higher energy. The isomer with trans terminal Br and bridging Cl ions has a lower energy and is more stable as the large and electron-rich Br are placed at terminal positions, therefore less electron repulsion than in bridging position. Also, more electronegative ligands increase tendancy to higher coordination numbers, which explain the relative stability of the two isomers.
Correct calculation, good accuracy of final energy value and nice justification of the relative stability! Just note, the complexes both have the same 'ligands' overall in terms of electronegativity so are actually equal in this respect. Smf115 (talk) 08:13, 22 May 2019 (BST)
AlCl2Br
Summary
Method and Basis Set: RB3LYP/Gen
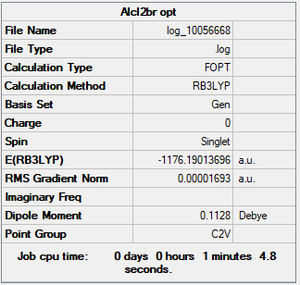
Optimisation
Item Value Threshold Converged?
Maximum Force 0.000036 0.000450 YES
RMS Force 0.000023 0.000300 YES
Maximum Displacement 0.000263 0.001800 YES
RMS Displacement 0.000154 0.001200 YES
Link: File:Angus Al opt freq.gjf.log
Frequency Analysis
Low frequencies --- -3.2441 -0.0025 -0.0013 0.0005 0.7695 2.3428 Low frequencies --- 120.4978 133.8276 185.7207
Rotatable 3d image of AlCl2Br
Dissociation Energy for lowest energy conformer to AlCl2Br
- E(AlCl2Br)= -1176.19014 a.u.
- E(Al2Cl4Br2)= -2352.41629 a.u.
- ΔE=-E(Al2Cl4Br2)+[2*E(AlCl2Br)]
ΔE=[2*-1176.19014-(-2352.41629)]a.u. ΔE=0.03601 a.u. ΔE=95 kJ/mol
Al2Cl4Br2 is more stable than the monomer as energy is needed to put in when breaking the Al-Br bonds. Also, it is easier to break a C-Br bond than a C-Cl bond due to poorer overlap of orbitals. Orbitals in Br are more diffused.
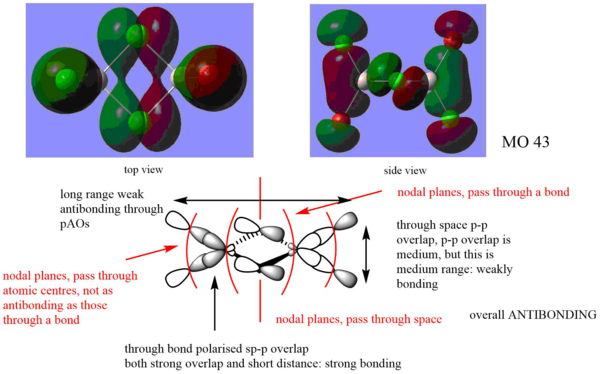
MO analysis on the lowest energy isomer
From the MO analysis of this conformer, there is a significant jump in energy from the core to the valence orbitals from MO30 to MO31. Therefore the MO 31 to MO59 (lowest 5 unoccupied MOs) were visualised and the following three MOs were chosen to do further analysis.
Clearly presented MOs using the two complementary screenshots and LCAOs. You've correctly identified the main interactions but there are a few mistakes, especially when evaluating the strength of the interaction e.g. the 'long-range weak antibonding' interaction between the two trans groups, the distance between them is so large that there is no evidential overlap. For the bottom two MOs, you also need to consider whether there is any contribution from the Al at all? Overall though, a good attempt at the analysis and evaluating the MO character. Smf115 (talk) 08:47, 22 May 2019 (BST)
A good report, well done! Smf115 (talk) 08:47, 22 May 2019 (BST)
Analysis of a highly bonding MO

Analysis of a weakly bonding MO

Analysis of a highly antibonding MO