Organic:whoisthedaddy
=Module 1=
The Hydrogenation of Cyclopentadiene Dimer
The Dimers shown to the right were modelled in ChemBio Ultra and their energies we're minimised using the Allinger MM2 porgram. Allthough the values obtained have little use on their own, they can be used effectively as a comparison between two simillar molecules.

After minimiseation the total energy of the Exo isomer is 133.4 kJ/mol which is slightly lower compared to that of the Endo isomer which is 142.3 kJ/mol. We know that the kinetic product (2) dominates in this reaction. However it is clear from the energy values that the Exo isomer is the thermdynamic product as it has the lower relative energy, this is due to the lack of steric hindrance between the two hydrogen atoms and the bridging carbon atom.
The same technique was the applied to isomer 3 and 4 to evaluate which double bond is easier to hydrogenate by comparing their relative energies.
In order to analyse this data knowledge about the hydrogenation mechanism must be revoked. The H2 molecule interacts with the surface of the hetreogenous Pt catalyst and the strong H-H sigma bond is broken to form two weaker Pt-H bonds. The Pi bond of the alkene then interacts with the metal catalyst which weakens the bond and allows two new C-H bonds to be form resulting in the formation of an alkane. This shows that steric hindrance will play a large part in the thermodynamic aspects of the reaction as the alkene needs to line up and interact with the solid catalyst.
Therefore the endo molecule with the smallest energy difference compared to the original endo cyclodiene will be the thermodynamically most stable, this is molecule A with only a 4kJ/mol difference compared to molecule B with a 20.1kJ/mol difference. This is due to the double bond in the six membered ring being much more sterically hindered than the double bond in the 5 membered ring. The bending values for molecule B is almost half than that of the value for A, this is due to the increased bending of the bridging C-C bonds by the double bond. Molecule D has a high Torsion value (52.0 kJ/mol), this is due to it's double bond is present in a five memebered ring compared to the lower torsion value (11.1 kJ/mol) for molecule C which contains the double bond in a six membered ring.
| Type | Energy (kJ/mol) |
|---|---|
| Stretch | 5.3 |
| Bend | 80.0 |
| Stretch-Bend | -3.5 |
| Torsion | 11.1 |
| Non 1,4 VDW | -6.8 |
| 1,4 VDW | 24.2 |
| Dipole-dipole | 0.7 |
| Total Energy | 146.3 |
| Type | Energy (kJ/mol) |
|---|---|
| Stretch | 4.8 |
| Bend | 54.4 |
| Stretch-Bend | -2.4 |
| Torsion | 52.0 |
| Non 1,4 VDW | -5.5 |
| 1,4 VDW | 18.6 |
| Dipole-dipole | 0.6 |
| Total Energy | 122.4 |
Stereochemistry of Nucleophilic additions to a pyridinium ring
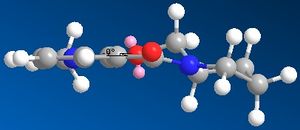

This same technique will now be employed to study the geometries and energies of two new examples. The first of which is shown to the left.
The energy of molecule 1 was minimised using molecular mechanics. This minimised structure was the used to gain certain measurements about the orientation of the carbonyl group in order to explain the stereoselectivity of the shown reaction. Primarily it must be explained that addition is onto the 4-position of the aromatic heterocycle, this is due to it being the most nucleophilic carbon atom (this can be shown easily by drawing resonance forms but is beyond the scope of this investigation).
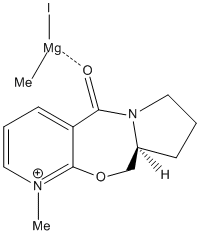
When viewing molecule 1 in its minimised forms it is clear to see that the top carbonyl group is orientated slightly above the plane of the molecule as shown in the diagram to the right. When measuring the dihedral angle of the most common minimised form of the molecule the carbonyl is 9° above the plane of the aromatic ring and with an energy of 175kJ/mol, this is quantitively explained in the diagram to the left. The other possible minimisation left the molecule with a dihedral angle of 23°, however this conformation gave a greater energy of 193kJ/mol. Various other orientations were attempted but all left the molecule with distortions which gave a much greater energy and therefore would not exist. These results are in agreement with the literature,1 which states that only two different orientations of the carbonyl group occur and they are both situated above the plane of the aromatic ring. Co-ordination between the amide carbonyl group and the magnesium of the Grignard reagent (see intermediate diagram) directs the methyl group to the front of the molecule. When comparing the energies of the two possible products, the expected product with the methyl group above the plane of the molecule is 0.1 kJ/mol lower than the molecule with the opposite stereochemistry of the methyl group. Which show that the product shown in the diagram above does actually occur.


When modelling the second molecule using ChemBio Ultra it is again clear that in it's minimised form the amide carbonyl is situated behind the plane of the aromatic ring by approximately 20°. The lone pair situated on the amine nitrogen atom will attack the most nucleophilic part of the aromatic ring which happens to be the 4C position due to reasons explained earlier. The stereochemistry of the product is determined by steric factors due to the bulky phenyl groups situated on the amine. Therefore the amine adds to the front of the molecule (opposite face to the carbonyl group) as shown in the diagram to the right. In order to improve this model I would include energy factors the chelation of the Mg atom into the calculation, this would increase the accuracy of the energy minimisation.
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol

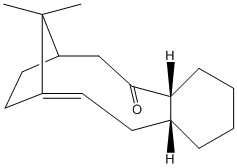
The two atropisomers were drawn and minimised using molecular dynamics. After attempting several different ring conformations (boat, chair and twist boat) it was found that the chair conformation was the most stable. This was found by manually converting between the different conformations and then minimising the energy . Molcule 11 (diagram to the right) was found to be the most thermodynamically stable product with a total energy 204.6 kJ/mol compared to 222.0 kJ/mol of molecule 10 (diagram to the left). Therefore I can predict that molecule 10 is the kinetic product, then after a certain period of time converts into molecule 11.

The alkene in both these molecule violates Bredt's rule2, which states that a double bond cannot be placed at the bridgehead of a bridge ring system3. The double bond is allowed due to the large natured of the ring system. The fact that it is placed next to a bridgehead of a bridged ring system lead to it becoming a hyperstable alkene4. This occurs when the Olefin Strain Energy of the alkene is lower than that of its corresponding alkane, this explains its slow reactivity.
Regieoselective Addition of Dichlorocarbene
During this experiment the molecule shown to the left will be modelled using the ChemBio Ultra program, minimised using the MM2 and MOPAC forcefields and the orbitals will then be calculated in order to understand its reactivity. The HOMO orbital is shown in diagram X.

It is clear from this diagram of the HOMO orbital that there is a difference in electron density between the two double bonds. The alkene on the left has a greater electron density and is therefore more susceptible to electrophilic attack as these electrons can interact with the LUMO from another molecule. This is due to the electronegativity of the Chlorine atom removing electron density from the right side of the molecule.
A Gaussian calculation was run on the original molecule and its mono hydrogenated form with the less electrophilic alkene removed, shown in the diagram to the right.
Guassian was used to calculate the IR frequencies for the C=C and C-Cl vibrations which are as follows;
Diene
C=C 1737.1 cm-1
C=C 1757.5 cm-1
C-Cl 770.8 cm-1
Monoene C=C 1758.1 cm-1 C-Cl 774.97 cm-1
The IR stretching frequency for the C-Cl bond diene is approximately 4cm-1 lower than that of the C-Cl bond for the monoene, this is due to interactions between the Π orbital of the additional double bond and the vacant sigma * orbital of the C-Cl bond. An increase in antibonding character of a bond causes it to become shorter and weaken.
The Diene C-Cl vibration is displayed below.
Characterisation of Products from the Reformatsky Reaction
A Reformatsky5 reaction is the formation of ester-stabilized organozinc reagents and their addition to carbonyl compounds. The example chosen involves a tandem chain extension-adol reaction by which B keto esters are transformed to A-substituted y keto esters in a zinc mediated reaction. This investigation will attempt characterise the products of the Reformatsky reaction shown in the diagram below.
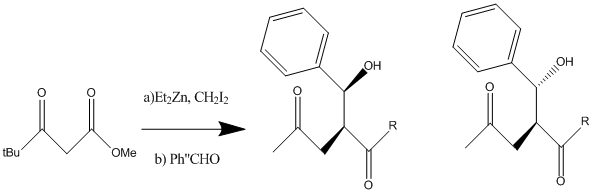
Both products shown in diagram X were minimised using the MM2 and MOPAC forcefields, they were then run through the Gaussian program in order to obtain spectroscopic information. However when these molecules were run through the guassian program the optimised molecule became extremely distorted and could not be used in any subsequent calculations. This is a limitation of this method as molecuels with a large amount of single bonds cannot be optimised due to free rotation and therefore the large amount of possible conformations.
Stereoselective dissolving Metal Reduction
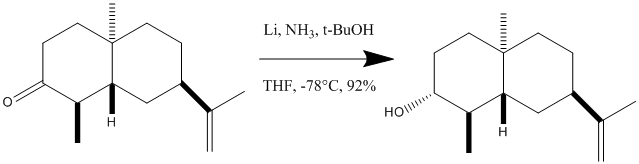
During the recent isolation of a new sesquiterpene amine6 the stereoselective Birch reduction of a cyclic ketone to an alcohol occurs and produces the stereochemistry shown to the right. The skills that have been developed so far in this investigation will be used to confirm that the product shown is correct and will characterise the product fully. Both possible isomers of the product will be minimised using the MM2 and MOPAC forcefields, this optimised geometry will then be run through Gaussian to obtain spectroscopic information on the two isomers.
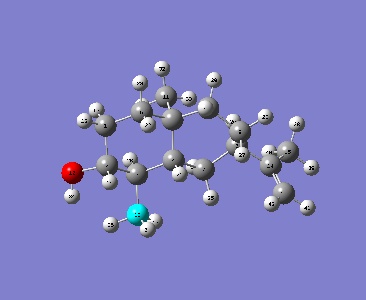

The NMR values (DOI:10042/to- ) were computed by the Gaussian and the resutls are shown below accompanied by the carbon labels in the image on the left. It is clear from this table that the nmr values differ slightly, therefore a reassignment of the C13 spectra must be carried out.
The appearance of the -OH vibration at 3825cm-1 as shown in the diagram to the right proves the reaction has occurred and the correct product is present, there is no IR value reported for the journal.
The reported optical rotation values are shwon in the table below. It can be seen that the two values for the -OH group facing down are in slight agreement, in order to obtain a more accurate calculation the orientation of the molecule would have to be modified manually.
Using the Karplus equation7 J3 values for both possible isomers were calculated and are shown in the table below. The simmilarity between the two values with the -OH group facing down are clear evidence that in the actual product the -OH group is in fact below the plane of the molecule.
| Orientation | Optical Rotation (°) |
|---|---|
| Down (journal) | -11.6 |
| Down (calculation) | -20.57 |
| Up (calculation) | 32.19 |
| Orientation | J3 Value (Hz) |
|---|---|
| Down (journal) | 13.2 |
| Down (calculation) | 11.5 |
| Up (calculation) | 3.7 |
| No of C | Down Calculated | Literature Value | Up Calculated |
|---|---|---|---|
| 1 | 29.54 | 30.92 | 28.82 |
| 2 | 13.08 | 39.93 | 16.43 |
| 3 | 18.08 | 22.82 | 23.79 |
| 4 | 19.31 | 23.08 | 25.38 |
| 5 | 28.95 | 26.05 | 28.01 |
| 6 | 21.97 | 38.88 | 20.23 |
| 7 | 27.58 | 33.78 | 26.22 |
| 8 | 25.48 | 43.22 | 20.31 |
| 9 | 31.84 | 37.1 | 27.37 |
| 10 | 25.76 | 39.19 | 19.44 |
| 11 | 91.49 | 76.86 | 96.57 |
| 13 | 139.1 | 144.43 | |
| 14 | 2.53 | 7.63 | |
| 15 | 63.43 | 57.55 | 147.11 |
| 16 | 11.29 | 110.67 | 12.7 |
References
1. A. G. Shultz, L. Flood and J. P. Springer; J. Org. Chemistry, 1986, 51, 838.
2. J. Bredt, H. Thouet and J. Schnitz; Liebigs Ann, 1924, 1, 437.
3. Pelayo Camps, Francesc Pérez and Santiago Vázquez; Tetrahedron, 1996, 37, 8605.
4. Eric V. Anslyn, Dennis A. Dougherty; Modern physical organic chemistry, 2006, 139.
5. Sujen Lai, Charles K. Zercher,Jerry P. Jasinski; Org. Lett 2001, 26, 4169.
6. Leonardo Castellanos, Carmenza Duque, Jaime Rodríguez; Carlos Jiménez, Tetrahedron, 63, 2007, 1544
7. C.A.G. Haasnoot, F.A.A.M. DeLeeuw and C. Altona; Tetrahedron, 1980, 36, 2783.
