Organic:tutorial:meerwein
The Classic Meerwein Rearrangement
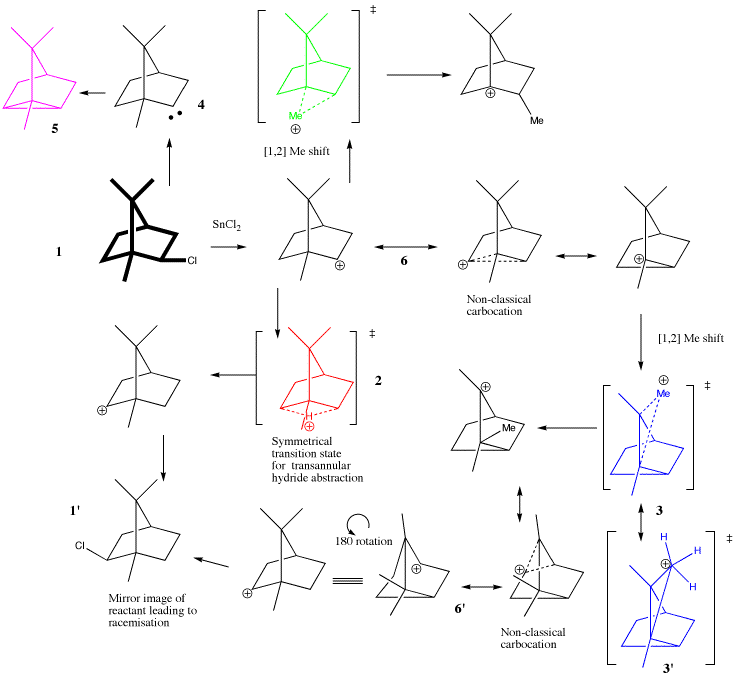
Meerwein's classic proposal of the mechanism for the racemisation of isobornyl chloride 1 in the presence of a Lewis acid led (eventually) to the widespread acceptance of carbocations (carbonium ions) as reactive intermediates in many organic reactions.1 The original suggestion by Meerwein in 1924 is equivalent to the proposal of the red transition state 2 (Scheme 1). Because this transition state has a plane of symmetry, it corresponds exactly to the inversion of one enantiomer of the initially formed carbocation to its mirror image. Nowadays, such a shift might be referred to as a transannular hydride abstraction by a carbocation.
Some nine years later, an alternative pathway was proposed2 (for a slightly different compound) involving instead a [1,2] methyl shift (blue transition state 3), which also leads to racemisation of the reactant 1. Such [1,2] shifts are nowadays known as Wagner-Meerwein shifts, although then they were referred to as Nametkin rearrangements. The transition state 3 also has a plane of symmetry, but it is not equivalent to the first pathway, since the carbon-carbon bond connectivity is not retained (as can be shown by isotopic labelling of the carbons).3
It has been known since 19533 that BOTH pathways can co-exist, and that the balance between them can be influenced by many factors. Nowadays, each pathway (plausible or otherwise) can be subjected to accurate quantum mechanical calculations, and by this means their relative energies can be estimated. When such calculations are carried out (B3LYP/6-31G(d)) the following results are obtained.
- The symmetrical red transition state has unusual geometric features. It corresponds more accurately to the sliding of a proton along the edge of a cyclopropane bond rather than to a hydride transfer between two classical carbonium ions (such a transfer would ideally would be expected to have the two carbon centres and the proton co-linear, which they clearly are not). The reactant-derived carbocation 6 and (mirror image) product carbocation 6' are not classical carbonium ions but instead are best considered as non-classical bridged species (and can also be considered as π-complexes between a carbocation and an alkene). The proton slide thus occurs between these two non-classical species, and hence should not be considered as a conventional (hydride) transfer. This transition state can also be considered as a edge protonated version of tricyclene 5, formed from the carbene 4.4 This particular route (the Tiffeneau divalent carbon hypothesis of 1907), was famously discredited by the Meerwein experiments. Whilst 5 as an intermediate is clearly wrong, an edge protonated 5 as a transition state is quite acceptable!
- The blue transition state corresponds to a more normal [1,2] methyl shift occuring in a more classical carbocation. It can also be more radically considered in similar terms to 5, namely as a protonated cyclopropane 3', but with the protonation occuring on a corner of the cyclopropane rather than on an edge. At the B3LYP/6-311G(3d,p) level of theory, this transition state is 7.6 kcal/mol lower in free energy than the red transition state. Factors such as solvation and counter ion interactions, and inaaccuracies in the theory could easily amount to this difference in energy.
- A pathway which does not occur is via the green transition state, corresponding to a [1,2] methyl shift to give a tertiary carbonium ion. It is now known (but it was not yet recognised in 1924-33) that although tertiary carbonium ions are more stable than secondary ones, this is not true if they occupy a bridgehead position, in which the carbocation cannot attain planarity. The calculated energy of the green transition state is indeed 43 kcal/mol higher than the red one.
The modern calculations can be seen to cast an interesting new light on these classic experiments, which represent the dawn of the age of mechanistic chemistry. The Meerwein shift, nowadays associated with [1,2] sigmatropic migrations, was in fact in this reaction actually a hydride migration with a very non-classical delocalized electronic structure. Even the initially formed carbocation 6, in 1924 then a very new and radical alternative to the previous wisdom of "divalent carbon" intermediates, itself became the subject of protracted controversy concerning its own non-classical nature. Indeed, one may conclude that very little about this fascinating problem is "classical".
| The Classic Meerwein Rearrangement | |||||
|---|---|---|---|---|---|
| The Meerwein-Montefort Mechanism (Red transition state -391.111445659/-390.900003 ) |
The Houben-Pfankuch Mechanism (Blue transition state -391.12578/-390.91217) | ||||
| The unobserved reaction (Green Transition state -390.92579/-390.711806 ) | |||||
References
- H. Meerwein and E. Montefort, Ann., 1924, 435, 207. For a fascinating analysis of this discovery, see J. Berson, "Chemical Creativity", 1999, Wiley, pp 116-136.
- J. Houben and E. Pfankuch, Ann., 1933, 501, 219.
- J. D. Roberts and J. A. Yancey, J. Am. Chem. Soc., 1953, 75, 3165. DOI:10.1021/ja01109a037 ; W. R. Vaughan and R. Perry, ibid, 3168. DOI:10.1021/ja01109a038
- K. Wolfram, L. Bowen and P. v. R. Schleyer, J. Am. Chem. Soc., 1989, 111 3479 - 3480; DOI:10.1021/ja00191a082 ; M. Lajunen, J. Chem. Soc., Perkin Trans. 2, 1986, 1551 - 1555, DOI:10.1039/P29860001551 K.-T. Liu, Tetrahedron Lett., 1978, 1129-32. DOI:10.1016/S0040-4039(01)94479-X
(C) Henry S. Rzepa, June 1, 2006.