Organic:ngp
An Example of Neighbouring Group Participation in Reactions of similar molecularity
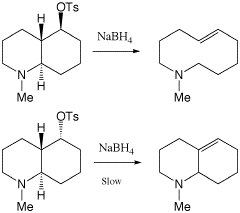
If the reactions shown on the right proceed by an initial loss of the Tosyl group (E1) then the rate of the reaction will not depend on the concentration of base (or indeed the basicity of BH4), but on how efficiently the Tosyl group can be expelled. The question then becomes whether the expulsion of the Tosyl group is assisted in any way by another group present in the same molecule, i.e. participation by a neightbouring group. It is at this point that the alignment of the remote nitrogen lone pair with respect to the cleaving C-OTs bond is crucial. If the two decalin diastereoisomers differ in this alignment, then the unimolecular rate constant k for loss of tosyl will differ for the two reactions. A good antiperiplanar alignment will increase the rate constant k (reduce ΔG‡ by reducing ΔH‡) with respect to a less efficient alignment. In the top reaction, the forming carbocation is in effect neutralised by concomitant donation from the lone pair on the nitrogen. In the bottom reaction, the aligment required to make this happen cannot be achieved, and hence no stabilisation of the carbocation can occur.
The conclusion that the alignment of the nitrogen lone pair is crucial is reinforced by the observation that the cis-decalin diastereomer does not undergo either of the two reactions shown here.
