Organic:entropy
The role of Entropy for Reactions of different molecularity
The fundemantal equations for understanding rates of reactions are
- ΔG‡ = ΔH‡ - TΔS‡
- ΔG‡ = -RT Ln k
In equation 2, k is the rate constant for the reaction. From this, you can see that the rate of a reaction depends on the entropy of activation for that reaction, ΔS‡.
For a unimolecular reaction of the type:
A ⇒ B + C
there are more degrees of freedom on the rhs than the lhs (the two molecules B and C can translate and rotate with respect to each other freely). ΔS‡ for such a reaction is therefore +ve. Plugged into equation 1, this reduces ΔG‡.
For an alternative bimolecular reaction invoking a base of this type;
A + Base ⇒ BaseH + D
there are more or less an equal number of degrees of freedom on both sides of this equation, and hence ΔS‡ for such a reaction is therefore close to zero. ΔG‡ in equation 1 is therefore not reduced. If ΔH‡ is the same for both reactions, then the unimolecular form will always be much faster than the bimolecular form (by a factor of 105 or so).
If the reaction takes the form
A + Base ⇒ BaseH+ + D + E
the degrees of freedom are again increased on the rhs, with again a +ve entropy of reaction. In these circumstances, the bimolecular reaction will not be at an entropic disadvantage compared to the unimolecular variation.
An unusual example of an Entropy Burst
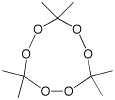
Equation 1 above implies that any reaction which increases the entropy substantially by formation of many more separate molecules in the products than are present in the reactant will favour exothermicity. An example of such an entropy burst reaction is the decomposition of the triperoxide of propanone giving three molecules of propanone and one of ozone, an increase in the molecule count of three. The enthalpy of this reaction is actually more or less neutral, and the decomposition is driven entirely by the free energy liberated by the entropy burst.
Reference
- F. Dubnikova, R. Kosloff, J. Almog, Y. Zeiri, R. Boese, H. Itzhaky, A. Alt, and E. Keinan, Decomposition of Triacetone Triperoxide Is an Entropic Explosion, J. Am. Chem. Soc., 2005, 127, 1146 - 1159. DOI:10.1021/ja0464903 .
