Organic:clubbanger
Module 1 - Organic
The Hydrogenation of Cyclopentadiene Dimer
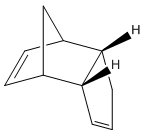
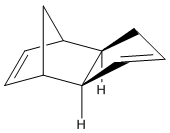
The dimerisation of cyclopentadiene occurs at room temperature, explicitly forming the endo isomer (top right), rather than the exo isomer (bottom right).
A computational analysis of the two dimers was run to compare their absolute energies. The calculations were done through a Molecular Mechanics Approach using the ChemBio3D Ultra 12.0 program. The energies were computed in the MM2 force field and the results for the two molecules shown to the right are tabulated below.
| Isomer | Energy / kJ/mol |
|---|---|
| Endo | 142.4 |
| Exo | 133.5 |
The table above shows that the energy of the favourably formed endo isomer is actually higher than that of the exo isomer. This implies that the process by which the dimer is formed must be under kinetic control as otherwise the lower energy, more thermodynamically stable, isomer would prevail.
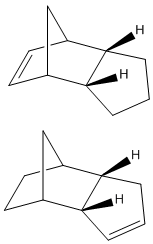
The image to the left shows the two partially hydrogenated forms of the cyclopentadiene dimers, the lower energy molecule retains the alkene bond in the 5-membered ring.
| Molecule | Total Energy | 1,4 VdW | Strain | Bending | Torsion | H-bonding |
|---|---|---|---|---|---|---|
| 6-membered hydrogd | 130.4 | 18.9 | 4.6 | 60.7 | 52.3 | 0.6 |
| 5-membered hydrogd | 149.3 | 23.6 | 5.3 | 82.9 | 45.5 | 0.7 |
Stereochemistry of Nucleophilic additions to a pyridinium ring
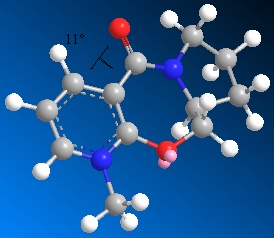
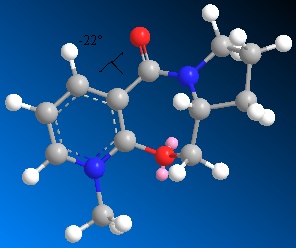
Molecules 5 and 6 shown to the right were optimised to a minimum energy using the MM2 force field. The geometries of the structures were then analysed to explain the stereo control observed in reactions with nucleophiles such as Grignard reagents.

Molecule 5, shown left, was optimised from several different starting geometries and patricularly attention was paid to the relative orientation of the carbonyl group to the pyridine ring. The mechanism of reaction of the nucleophile at the 4-position of the pyridine ring involves the magnesium's coordination to the carbonyl oxygen, this strong interaction directs the methyl group to the same face of the ring as the carbonyl is on, so the relative geometry of this group will be a key factor in the stereochemistry of the product1.
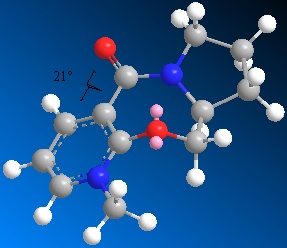
Optimisations of molecule 5 led to 3 particular dihedral angles (1-2-3-4 shown on the diagram) of the carbonyl group with the pyridine ring. Most optimised to a dihedral of around 22deg. but one optimised to a slightly smaller angle of 12deg. There was also one optimisation which led to a negative dihedral which the literature had implied would not occur - the angle being -22deg. The predominance of the slighlty positive dihedral angles between these two groups explains the stereocontrol in the reaction with the Grignard nucleophile. The methyl is known to add on the top face of the ring, as drawn, and this would allow the magnesium to coordinate with oxygen during the addition and so lower the energy of the transition state.

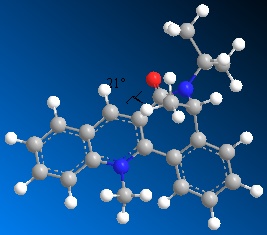
Molecule 6, shown left, optimises to a minimum with the dihedral angle (1-2-3-4) between the carbonyl and the pyridine ring as negative. The magnitude is similar to in molecule 5 with the dihedral optimal at ~-20deg. Negative in this case implies that the carbonyl is on the opposite face of the ring to the methyl group also on the 7-membered ring. The effect this has on the stereochemistry of the addition product is mainly due to the interactions of the oxygen's and incoming ammine's lone pairs. The repulsive coulombic forces between these lone pairs causes the ammine to add to the opposite face as the carbonyl. The molecule was optimised from several different starting positions, all leading to the minimum with dihedral angle of ~-20. This implies that the most stable (low energy) conformations of molecule 7 all have the carbonyl oxygen slightly below the ring, which therefore leads to the product being very specific in having the ammine add on the top face of the pyridine ring.
The dihedral angle was also constrained to a positive value around +20deg. to gain a value for the difference in energy from the optimal conformation. The result showed a molecular energy ~40kJ/mol higher than the suspected global minimum.
The models used for this analysis could certainly be improved by using a higher level of theory. The MM2 force field is a purely mechanics based model and so one with at least a partial quantum element would improve the analysis as the electronic structure and orbital interactions would be taken into account to some extent. Apart from this more obvious altercation to the method, the MM2 can be improved by changing the parameters on which it is based to systems more similar to the one being studied.
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
Optimisation of the intermediate in the synthesis of Taxol gave two distinct conformers as shown below.

The key difference between the two conformers is the orientation of the carbonyl group relative to the ring. The left hand image shows the oxygen pointing slightly down and out of the 10-membered ring, whereas the right hand image shows the carbonyl pointing up and into the ring. The carbonyl up and in is a lower energy conformation at 204.1kJ/mol compared to 213.1kJ/mol for the 'down and out' conformer. The difference in energy of 9kJ/mol indicates that the orientation of the carbonyl up and into the ring is the most stable conformation. The optimisations of the molecule were slightly challenging due to the large, flexible 10-membered ring. This creates a large amount of conformations that the molecule can adopt, many of which will be similar in energy - i.e the PE surface will be relatively flat. This in turn causes problems for the optimisation as the minimums will not be so clear cut as in a more constrained molecule. The initial optimisations were problematic, and would give structures with over-lapping atoms that were clearly unstable; manual manipulation of the groups to a more realistic position then allowed the structure to form a more sensible looking conformation. The calculations would also sometimes run for a relatively long time with the atoms move rather large distances on each step. This was indicative of the large flat PE surface and again, some manipulation of the atomic positions helped the molecule optimise. The final structures obtained look to be stable, with large distances between non-bonded atoms and roughly tetrahedral geometry around each sp3 carbon.
The conformation of the cyclohexane ring was also seen to have an effect on the overall energy of the molecule. Initial optimisations led to a twisted boat conformation in this ring - both for the carbonyl up and down conformations - but a quite signifficantly lower (~20kJ/mol) energy was obtained from manipulating the 6-membered ring into a chair conformation and then reoptimising from this point.
The observed slow reactivity of the alkene in the molecule shown above is suspected to be due to increased transannular and vicinal hydrogen interactions around the ring in the saturated form of the molecule. This inplies that any substituents added across the double bond will greatly increase the strain in the molecule and possibly make the addition energetically unfavourable. This is the thermodynamic interpretation of the observed stability and is referred to in the literature as 'hyperstable olefins'. It is also possible that a kinetic barrier is the reason behind the non-reactivity, but it is not immediately obvious where this would come from as the alkene is fairly unhindered. 2
Regioselective Addition of Dichlorocarbene
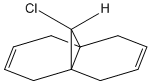
The computational analysis of a bicyclic diene - shown left - was undertaken to analyse the regioselectivity of electrophilic addition to the molecule. Initially the molecule was optimised using an MM2 force field, the resultant structure was then optimised again using a semi-empirical method - MOPAC/PM6. This gave some electronic information on the molecule and also allowed MOs to be visualised. Images of the relevant orbitals are shown to the right.
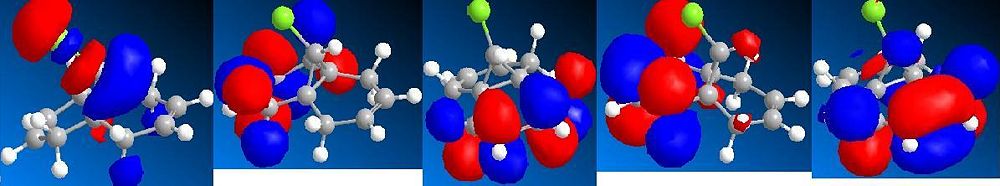
The HOMO shows a majority of the electron density on the alkene bond endo to the chlorine. This makes this double bond more susceptible to electrophilic attack as it contains electrons in the highest energy occupied orbital so will presumably be the most prone to interaction with empty orbitals on other molecules. The method did discriminate between the two alkene bonds, as the HOMO and LUMO are seen to be based separately on the different alkenes.
Information on the stretching frequency of key bonds in the diene was also required and this was obtained using a method based on density fuctional theory B3LYP with the basis set of 6-31G(d,p). The extra diffuse orbitals included for each atom (signified by the d and p in the brackets) allow a more accurate representation of the electronic structure and therefore the conformation of the molecule. The data for the vibrational frequencies were analysed and those corresponding to the C=C and C-Cl stretches are tabulated below.
| Bond | Frequency |
|---|---|
| C-Cl | 771 |
| C=C (exo to Cl) | 1737 |
| C=C (endo to Cl) | 1757 |
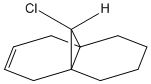
As well as this diene, an analysis of a partially hydrogenated form was also undertaken so as to view the changes in the bond stretching frequencies. The structure of the hydrogenated form is given to the left and the table of data is below, it now contains just one double bond - endo to the Cl on the bridging carbon.
| Bond | Frequency |
|---|---|
| C-Cl | 780 |
| C=C | 1754 |
The frequencies of the two bonds change upon the hydrogenation of the endo alkene, the C-Cl frequency is seen to increase from 770 to 780cm-1 - indicating an increase in bond strength - and the C=C bond decreases slightly from 1757 to 1754cm-1. The reasons for these changes are expected to have their origin in orbital interactions. The C-Cl sigma* orbital has a large lobe on the carbon, pointing towards the endo alkene bond. When this bond is present, some electron density from the pi-cloud can be donated into this empty sigma* orbital. This has the effect of lengthening the bond, as the effective bond order decreases and it becomes weaker. This lengthening is seen in the lower frequency absorbed in the bonds vibrations compared to the hydrogenated molecules data. In the hydrogenated molecule the pi-cloud is not present and so no electron density is available to be transferred into the C-Cl sigma* and the bond is shorter, stronger and absorbs at a higher frequency. It is not immediately obvious why the C=C stretching frequency changes by 3cm-1.
Spectroscopic Analysis of Diastereoisomers of two Tetrahydrofuran Derivatives
A paper in the Journal of Organic Chemistry in 20043 discussed, in part, the two diastereoisomers formed from the intramolecular cyclisation shown below.
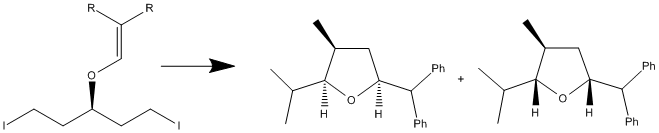
The cyclisation proceeds via radical intermediates and was reported in the paper to form the two products in a ~1:1 ratio. A computational analysis was undertaken to compare the spectroscopic data of the two isomers and this started with an optimisation in the MM2 force field. This optimised structure was then immediately submitted to optimisation under a semi-empirical method - MOPAC/PM6. These initial calculations were simply done to reduce the amount of CPU time required to optimise the molecule using Density Functional Theory based methods, namely B3LYP. The optimised structures of the two isomers are shown below.
As spectroscopic data will be collected on these two isomers it is necessary to identify the key differences expected in the data for each. Looking at the structures, one of the key changes may be in the methyl groups C13 NMR peak, as it quite visibly canges environment between the two isomers.
Unfortunately, the supporting information for the paper this reaction appeared in was not available and therefore there was not the required comprehensive spectroscopic data.
Another suitable reaction was chosen to replace the above, this was found in the Journal of Organic Chemistry in a paper submitted in 20034. The reaction is given below, and involves the hydrogenation of an olefin to give two diastereoisomers. These two conformers will both be subjected to a computational analysis to compare aspects of their spectroscopic data.
The two products - of which the ester group pointing forward is the major - were initially optimised in the MM2 force field, followed by the semi-empirical method MOPAC/PM6. This was then furthered with an optimisation at the higher level of theory b3lyp, which uses Density Functional Theory; the basis set was 6-31G(d,p). The optimised structures are shown below. A frequency analysis of the two conformations was undertaken to check that the optimisations had indeed proceeded to a minimal stationary point. This was the case, signified by all of the frequencies being positive.
| Frequency | Ester Forward (major) | Ester Back (minor) |
|---|---|---|
| 1 | 2968 | 2968 |
| 2 | 2938 | 2937 |
| 3 | 1726 | 1727 |
| 4 | 1704 | 1706 |
| 5 | 1155 | 1115 |
| Peak | Ester Forward (major) | Ester Back (minor) |
|---|---|---|
| 1 | 2.74-2.63 | 2.56-2.46 |
| 2 | 2.67-2.59 | 2.48-2.29 |
| 3 | 2.38-2.22 | 2.08-1.4 |
| 4 | 2.16-2.08 | 0.94 |
| 5 | 2.05-1.4 | |
| 6 | 0.92 |
| Peak | Ester Forward - Reported | Ester Back - Reported |
|---|---|---|
| 1 | 214.7 | 213.6 |
| 2 | 175.4 | 174.4 |
| 3 | 79.9 | 80.1 |
| 4 | 61.6 | 60.6 |
| 5 | 43.9 | 45.3 |
| 6 | 42.2 | 41.2 |
| 7 | 37.8 | 38.4 |
| 8 | 35.2 | 33.4 |
| 9 | 34.8 | 30.5 |
| 10 | 29.7 | 29.3 |
| 11 | 28.8 | 28.0 |
| 12 | 28.0 | 28.0 |
| 13 | 22.9 | 24.3 |
| 14 | 15.9 | 16.1 |
The reaction is reported in the journal to have a preference (87:12) to form the 'Ester forward' compound, and the reasons for this can be seen in the mechanism of the reaction. The hydrogenation was catalysed by Pd/C with 1atm of H2. The reactent olefin adsorbs onto the palladium surface to initiate the reaction, and the hydrogens then add in a cis fashion across the double bond. By which face the reactant adsorbs onto the palladium defines which face the hydrogens add to, and therefore which face the ester group finishes on. The reason for a major and minor product must therefore lie in some discriminatory process for the reactant to adsorb via one face rather than the other. It is reported in the journal that the carbonyl on the 6-membered ring is responsible for the bias as the oxygen is coordinated to the methanol solvent and so this face is more hindered on approach to the catalyst.
The calculation at B3LYP/6-31G(d,p) gave a difference in energy between the two isomers at 4kJ/mol in favour of the ester forward which is the major product by a ratio of almost 9:1. This implies that the hydrogenation process must control the stereochemistry as this small difference in energy would not give rise to a such a bias in formation of the isomers. The hydrogenation process is irreversible under the conditions so is under kinetic control. As reported above, the kinetic barrier for adsorption onto one face of the olefin must be much lower than the other.
The images below show the 'Ester back' isomer on the left and the 'Ester forward' isomer on the right

| Carbon | Ester Back | Ester Forward |
|---|---|---|
| 6 | 201.6 | 201.8 |
| 13 | 162.6 | 161.6 |
| 16 | 72.0 | 71.7 |
| 5 | 53.2 | 54.5 |
| 10 | 41.0 | 41.1 |
| 4 | 34.5 | 33.7 |
| 1 | 30.3 | 30.7 |
| 9 | 25.3 | 26.4 |
| 7 | 29.0 | 25.7 |
| 8 | 22.6 | 24.7 |
| 3 | 23.2 | 23.7 |
| 18 | 16.8 | 21.9 |
| 19 | 17.1 | 17.2 |
| 2 | 18.9 | 18.9 |
| 17 | 21.8 | 16.9 |
| 12 | 9.2 | 8.9 |
| Bond | Ester Back | Ester Front |
|---|---|---|
| C-O | 1188, 1261 | 1301, 1200 |
| C=O (ketone) | 1799 | 1795 |
| C=O (ester) | 1797 | 1804 |
| C-H | 1100-1200 & 3000-3300 | 1100-1200 & 3000-3300 |
The spectroscopic data gained from the calculations on the two isomers agrees generally quite well with the reported data in the journal. Unfortunately, the NMR and IR values were not assigned to particular bonds or nuclei in the paper so some interpretation was required. Looking initally at the IR, the stand out difference between the calculated values and the reported values is that all of the calulated data is larger than the relative reported values. This is a known discrepency in gaussian calculations to over-estimate IR absorptions. Instead of looking at the absolute values, the relative values between the two isomers can be studied and these differences are similar in both the reported data and the calculated.
The single C-O bond stretches are particularly different between the two isomers, with the reported data at 1155 for the ester group forward and 1115 for the ester group back. These were the only peaks reported in this region, despite esters usually giving two distinct bands. The computational data gave two distinct stretches for each isomer and again the 'ester forward' isomer had larger values of 1301 and 1200 compared to 1261 and 1188 for the ester back molecule. Despite the differences in magnitude - as explained - the relative values for the computational analysis show good agreement with those from experimental data. The rest of the IR spectrum - a majority of which is C=O and C-H peaks - are very similar for both isomers in the computational and experimental data; meaning that the differences in the C-O stretch would have been a key difference the synthesisers would have looked for when assigning the separated isomers. As the computational data agrees with the experimental assignment, it is good evidence that the assignment of isomers is correct.
The 13C NMR data for both isomers is very similar both in the reported experimental data and the calculated computational data. This was expected as the two isomers are stereoisomers rather than regioisomers so the carbons are in almost exactly the same chemical environment. The minor differences that do occur though, seem to occur both in the experimental and computational data. The main difference observable is in the carbons on the 5-member ring adjacent to the carbon connected to the ester group (numbers 7 and 9 in images above). The computed chemical shifts for the ester forward isomer were 25.7ppm for C7 and 26.4 for C9. The ester back isomer were 29.0 for C7 and 25.3 for C9. This small change in the shift is due to the slight re-orientation of the ring to accomodate for the changing position of the ester and to ultimately minimise the conformations energy.
As the NMR shifts were not assigned in the paper, some interpretation was required. Again the magnitudes of the experimental and computational data differed, this time with the computational being an under-estimate. The reported shifts that are expected to be due to C7 and C9 are numbers 8 and 9 in the experimental table above. The values given in these were 35.2 and 34.8 for ester forward and 33.4 and 30.5 for ester back. As the data was not assigned by the experimentalists, no full conclusions can be drawn but there does seem to be some parallels between the data. The ester back isomer, in both cases, has a larger difference between the two carbons' shifts. This again implies that the original assignment of the isomers was correct.
Overall the agreement between experimental and computational seems to be quite good - taking into account the indiscrepencies of the calculation program. The differences between the two isomers are subtle and they would be quite to assign just on NMR and IR data given by the computational method.
References
1. A. G. Shultz, L. Flood and J. P. Springer, J. Org. Chemistry, 1986, 51, 838. 2. Cross-coupling of a functionalized highly pyramidalized alkene: DSC and NMR study of the [2+2] retrocycloaddition of cyclobutane cross products, hyperstability and pyramidalization of the formed dienes, Tetrahedron 57 2001 3. Robert Andrukiewicz, Piotr Cmoch, Anna Gaweł, and Krzysztof Staliński J. Org. Chem., 2004, 69 (6), pp 1844–1848 4. A Phosphine Catalysed [3+2] Cycloaddition Strategy leading to the First Total Synthesis of Hinosol, J. Org. Chem. Du, Y Lu, X - 2003