Organic:arrow
Examples of transient intermediates involved in Arrow pushing
The examples set out here illustrate how counting valence electrons associated with the atoms in molecules can indicate their likely stability. Thus as a very approximate rule of thumb, molecules where all the atoms have an octet of electrons (for main group elements, excluding hydrogen) tend to be bottle-able, whereas those where one atom may only have a reduced count (6 or 7) can be very reactive/unstable. It should be said that many of the examples below are isolable because the electron deficient atom overcomes this by one subterfuge or other! A set of interactive arrow pushing tutorials can be found here. A departmental blog post on the topic can be found here.The problems illustrated below are available ![]() here
here![]() or by clicking on the image File:FoundationTutorialproblems09.pdf.
or by clicking on the image File:FoundationTutorialproblems09.pdf.
Product of reaction between Et2O + MgBr2 → Et2O-MgBr2
Click to view The direct reaction product produces an apparently tri-coordinate Mg with a formal valence electron count of 6 at Mg. The actual shape is a more complex structure in which the valence deficiency at Mg is rectified not so much by adding two further valence electrons via a fourth ligand, but by involving two further bromine ligands from another molecule, which are each shared between two Mg atoms. The result is in fact an infinite polymer. What now is the valence electron count involving the Mg, which has almost exact trigonal bipyramidal geometry? (Hint, its pretty similar to eg PF5 and other row 2 main group elements with such trigonal geometry). Of course, this polymeric structure can be broken down if a further molecule of Et2O is added. However the resulting structure is not (R2O)2.MgBr2 but a more complex dimeric form with bridging bromines and octahedral magnesium. The dimeric form is also seen between diethyl ether and benzyl magnesium bromide Grignard, where now the magnesium is tetrahedrally coordinated. If in fact four ether molecules are added to MgBr2, an octahedral complex is formed (actually of thf4MgBr2). So Mg here is seen in tetrahedral, trigonal bipyramidal and octahedral coordination.
If you want a bit more fun, go see an analysis of the bonding in the related compound, diphenylmagnesium, or Ph2Mg.
Tetrahedral intermediate involved in Amide hydrolysis/Nucleophilic addition
Click to view Tetrahedral intermediate This is an example of a carbon surrounded by four different groups (and which is thus chiral), three of which are heteroatoms which themselves contain lone pairs of electrons. Any one of these lone pairs can in principle evict one of the other heteroatomic ligands, reversing the nucleophilic addition which produced the intermediate in the first stage. Notice how unequal the two C-O bond lengths are, one being shortened (at the expense of the C-N bond, which is lengthened). This is an example of a stereo-electronic effect, and these effects in large part are why this type of intermediate is often unstable, and hence not isolated during such reactions.
Other types of tetrahedral intermediates
Click to view Tetrahedral intermediate | Click to view lone pairs This first is of interest because it shows an organic compound not only as a tetrahedral intermediate, but as a di-enolate anion, with Na+ cations surrounded by water in a fascinating bridging motif. The enolate itself contains TWO tetrahedral centres. The model to the right includes the located positions of the lone pairs. See which of these you can align antiperiplanar to a C-X (X=O,N) bond!
Click to view crystal structure This second example is of (RO)2C(OH)2, a relatively rare example of a carbon with four oxygen ligands, two of which are OH. C(OH)4 itself (fully hydrated carbon dioxide) is unknown, but C(OR)4 is well known. Oh, if you know what an anomeric effect is, how many instances of this can you find in the molecule below? Note also how one of the C-O bonds is 1.51Å long. Another interesting question is why the two C(OH)2 groups prefer this form rather than the dehydrated one (a carbonate).
Wheland intermediate involved in aromatic electrophilic substitution
Click to view Wheland intermediate In this crystalline example, the electrophile is Cl-CH2+ with GaCl4- as the counterion, adding to hexamethylbenzene. The secret to trapping such intermediates is to inhibit their breakdown, which normally occurs by nucleophilic or basic abstraction of one of the two atoms on the sp3 centre. This can be achieved if the negative counter-ion is neither nucleophilic or basic. The design of such counter-ions is a highly active current research area.
By the way, although this intermediate is named after George Wheland, who studied the species by theoretical methods in the 1940s, an equally clear description of its properties was made by Henry Armstrong (working at what was to eventually become Imperial College London), back in 1890 (DOI:10.1039/PL8900600095 (see page 102, first paragraph), and a strong case could be made for calling it an Armstrong intermediate.
Carbocations
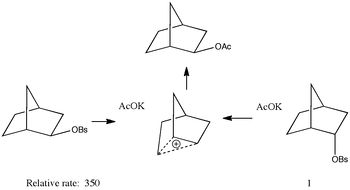
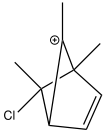
Click to view non-classical carbocation Solvolysis of brosylates (p-bromobenzenesulphonate) by e.g. acetate would be expected to result in SN2 inversion at the carbon bearing the leaving group. But in the example shown here, solvolysis of either the exo or the endo stereoisomer gives the same product, the former revealing apparent retention rather than inversion of configuration. This has been famously explained as involving a non-classical and symmetrical carbocation. In effect, the initial leaving group is evicted by the electron pair of a C-C bond in a Sn2 fashion, and then the acetate quenches the cation from the same side as the leaving group left from (for the exo reactant, ie overall a double inversion) or from the opposite side (for the endo reactant). For many years, this mechanism was highly controversial, but has been recently settled in its favour by (amongst other evidence) the crystal structure of the carbocation shown here. Some asymmetry is evident, but this is largely caused by the presence of methyl groups on some carbons and not others.
Another example of a carbocation shows it adjacent to an alkene group, with which it interacts. If you load the 3D structure you can see the distorsion in the structure caused by this neighbouring group effect.
Bromonium ion intermediate in the bromination of an alkene
Click to view Bromonium ion. The bromonium ion is normally not isolated because its subsequent attack by nucleophile (e.g. Br-) is so fast. In this example, this attack has been completely inhibited by steric effects. If you right mouse click in the molecule window, and from the pop up menu select style/scheme/CPK spacefill, you may observe something interesting! The structure below is interesting for another reason. It contains a hydrated proton, H3O+. Naked protons are never found in the solid or liquid state. This hydronium ion has three (very strong) hydrogen bonds to each of the triflate anion groups.
Nitronium and acylium cations
Click to view asylum ion | Click to view nitronium ion.
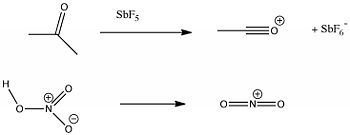
Aromatic electrophilic substitution is accomplished using cations which are generated from suitable precursors, and are never normally observed themselves. Here are two very common such species, the nitronium cation and the acylium cation. Notice how very short the bonds are in these cations; they are amongst the shortest bonds recorded. The counter-anions in these crystal structures are heavy metal chlorides or fluorides, Sb for the acylium cation, Mo for the nitronium cation (the later also has a molecule of acetonitrile solvent in the crystal which appears to be interacting with the nitronium ion).
Enolate intermediates in ketone alkylation
Click to view enolate intermediate
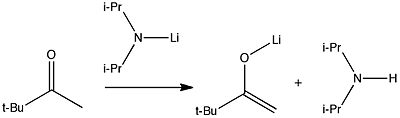
Ketones are alkylated by a strong base such as LDA removing a proton to form an intermediate enolate, which then acts as a nucleophile in attacking an electrophilic alkylating agent. If deprived of an electrophile, the intermediate enolate can be isolated, as shown below. In this case, the Li (the "L" in LDA) forms an interesting hexameric cage structure involving six separate molecules of the enolate.
Carbenes
Click to view carbene intermediate
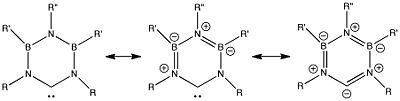
Carbenes are formally divalent carbon, of which only two of its four valence electrons are used for bonding. The other two are (formally) in a non-bonded lone pair (if the molecule is in its singlet electronic state). Carbenes are normally regarded as highly reactive intermediates, and their existence is inferred by trapping experiments (forming eg cyclopropanes etc). But recently, a new family of so-called stabilized carbenes have been isolated as stable species. Show below/right is one such, which has some other interesting features. The schematic diagram shows three resonance forms, two of which reveal the divalent carbon as a carbene, and the third is quite different! The structure is shown below, with the bond lengths indicated. Can you conclude from this which of the three resonance forms is actually the most realistic? And why?
A very recent postscript takes this concept to an extreme elaboration. If a carbon which uses only two of its four valence electrons for bonding is termed a carbene, then a carbon which uses none of its valence electrons for bonding would be called a carbone. Yes, absurd as it sounds, lots of examples of carbones have now been identified!
Nitrenes
Click to view nitrene intermediate
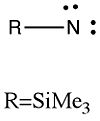
Nitrenes are the nitrogen equivalent of carbenes. Only one of the five valence electrons is used for bonding (formally), which leaves two lone pairs on the monovalent nitrogen atom. This is a very reactive species, and it can be readily trapped by all manner of substates. The one shown below is a Vanadium atom, itself ligated by two cyclopentadienyl ligands. An interesting (advanced) exercise is to count the valence electrons involving the Vanadium. Remember, with d-shell metals, the count is a total of 18 not 8 (i.e. 2 + 6 + 10 in the s, p and d-shells). Vanadium has an atomic electronic configuration of [Ar].3d3.4s2.4p0. Does it reach the magic 18 in this example?
Lithium alkyls
Click to view n-butyl lithium Lithium is a commonly used metal to form metal alkyls, normally with the alkyl group being either n-butyl or t-butyl. In the solid state both these molecules form oligomers; a hexamer for n-butyl and a tetramer for t-butyl. The structures of both are unusual and unexpected DOI:10.1002/anie.199305801 . These structures are especially important when deciding aspects of the reactivity of such species such as regio or stereselectivity.
More examples of the use of Crystal structures in teaching
The Cambridge crystallographic database centre offers further teaching resources based on a sub-set of their database, including slide shows for a recent workshop
- Using the Cambridge Structural Database to Introduce Important Inorganic Concepts, DOI:10.1021/ed079p1278
- Using the Cambridge Structural Database To Teach Molecular Geometry Concepts in Organic Chemistry DOI:10.1021/ed086p460
- Teaching Three-Dimensional Structural Chemistry Using Crystal Structure Databases. 1. An Interactive Web-Accessible Teaching Subset of the Cambridge Structural Database DOI:10.1021/ed100256k
- Teaching Three-Dimensional Structural Chemistry Using Crystal Structure Databases. 2. Teaching Units That Utilize an Interactive Web-Accessible Subset of the Cambridge Structural Database DOI:10.1021/ed100257t
