Organic:Victoria
The Hydrogenation of Cyclopentadiene Dimer
Isomers 1 and 2 are the exo and endo forms respectively of dimerised cyclopentadiene. Each if the two isomers were modelled on ChemDraw3D to produce a 3D representation of each molecule (an example of which is shown in figure 2). After 3D modelling Molecular Mechanics was used in order to optimise the engies of the structures(in this case MM2 was used, though the program does support other MM calculations). The result for molecule 1 gave an overall energy of 133.4KJ/mol compared to the value calculated for molcule 2, 142.3KJ/mol. This information shows that molecule 1 is the thermodynamic product as it is of lower energy and therefore more stable. Cyclopentadeine dimerises to produce solely the endo product, therefore the dimerisation of cyclopentadiene is a irreversible, kinetically controlled process.
| Energies | Exo Dimer | Endo Dimer | Molecule 3 | Molecule 4 |
|---|---|---|---|---|
| Stretch | 5.4 | 5.2 | 5.3 | 4.6 |
| Bend | 86.2 | 87.2 | 82.9 | 60.9 |
| Torsion | 32.0 | 39.8 | 45.5 | 52.3 |
| 1.4-VdW | 17.7 | 18.1 | 23.6 | 18.8 |
| Total Energy | 133.4 | 142.3 | 149.3 | 130.3 |
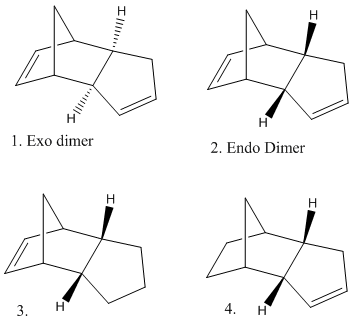
comments on why the exo product is the themodynamically more stable, sterics et?
Similarly the products of the hydrogenation of molecule 2 (the endo dimer) can be examined. Molecular Mechanics can be used in order to suggest a thermodynamic prediction of which double bond in the molecule will most easily be hydrogenated.Molecule 3 has an overall energy of 149.3KJ/mol whereas the energy of Molecule 4 is 130.3KJ/mol. These values show that molecule 4 is most thermodynamically stable. In addition tot he overall energy values the contributions from bending, stretching, van der waals, hydrogen bonding and torsion were examined. Both molecules 3 and 4 show a very small contribution from stretching (5.3 and 4.6KJ/mol respectivly) which implies the bonds are not very stretched from their 'normal' lengths. Both moleucles alos show an almost insignificant contribution from hydrogen bonding (0.7 and 0.6KJ/mol) this is because there are no atoms with a signifcant electronegativity present which is necarssary in order for hydrogen bonding to occur. Other contributions include bending, which molecule 3 shows a much larger contribution from (82.9 compared to 60.9KJmol). This suggests that the bonds in molecule 3 are bent more from their normal than the bonds in molcule 4, which will reduce their stablity. TORSION
Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+ analogue)
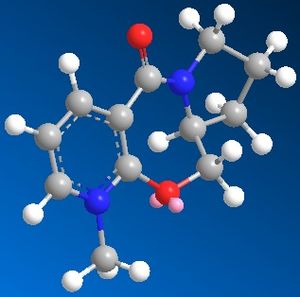
When the following molecule was modelled in 3D using ChemDraw and the energy minimised using MM2 calculations it was found that the preferable orientation of the carbonyl group was above the plane of the pyridiene ring.
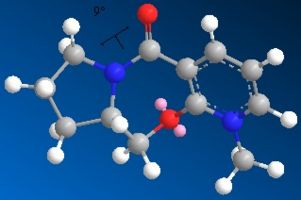
Whilst modelling the molecule it was found that depending on the orientation of the bonds to begin with had a significant effect on the result of the calculation carried out. That is, I found that from different orientations different energy minima would be found. As I was searching for the global minima of the conformer rather than the local minima it was necessary to manipulate the orientation of several bonds on the 3D model to eventually find the very lowest energy conformer. Following is the energy data relating to the global minima and one of the local minima I found the conformation was prone to return to without extreme manipulation of the 3D model.
| Energies | Dihedral Angle 12° | Dihedral Angle 24° |
|---|---|---|
| Stretch | 7.7 | 4.9 |
| Bend | 60.3 | 31.1 |
| Stretch-Bend | 0.6 | 0.4 |
| Torsion | 25.5 | 60.3 |
| Non VdW | -2.3 | -9.5 |
| 1.4-VdW | 70.3 | 67.7 |
| Charge/Dipole | 7.4 | 11.9 |
| Dipole/Dipole | -16.5 | -15.9 |
| Total Energy | 153.0 | 150.9 |
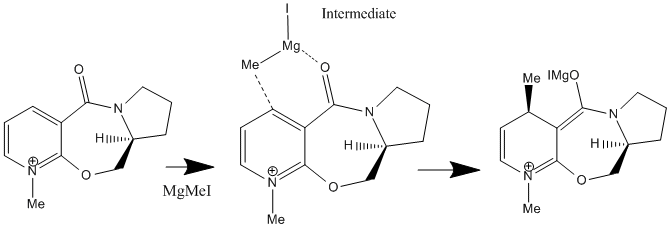
When this molecule reacts with methyl magnesium iodide, alkylating the pyridine ring in position 4 it is found that the product has the stereochemistry shown in (FIGURE?????), i.e. the methyl group is orientated up, it is above the plane of the pyridine ring. This can be explained best by the intermediate of the reaction. The magnesium of the Grignard reagent is small and hard, like the oxygen of the carbonyl group. The magnesium is also electropositive whilst the lone pair on the oxygen makes it electronegative. These factors cause the magnesium to coordinate to the carbonyl oxygen during the reaction forming the intermediate (FIGURE ???) which then delivers the methyl group onto the pyridine ring. From this coordination it is clear that the methyl group must be delivered on the same plane that the oxygen is on (due to the inability of the Grignard reagent to bend round to the other plane of the molecule). As discussed above , the most thermodynamically stable, and therefore the position the carbonyl group is found in, is pointing above the plane of the pyridine ring, in this reaction therefore the methyl group is directed above the plane of the pyridine ring also.
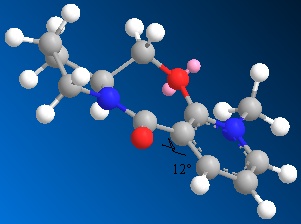
As with the previous molecule, molecule 2 was modelled in 3D using Chemdraw 3D and then the energy was minimised using MM2 calculations to find the most thermodynamically stable and therefore favourable conformer of the molecule. Many local minima were found for this molecule but from the datat it is clear that the thermodynamically most stable conformer has the carbonyl group orientated below the plane fo the pyridine ring.
Following is the energy data from a number of the minimum configurations found.
| Energies | Dihedral Angle 55° | Dihedral Angle -21° | Dihedral Angle -23° | Dihedral Angle -19° |
|---|---|---|---|---|
| Stretch | 16.4 | 16.5 | 15.9 | 16.6 |
| Bend | 48.9 | 49.3 | 47.1 | 49.4 |
| Stretch-Bend | 1.7 | 1.7 | 1.6 | 1.7 |
| Torsion | 41.1 | 39.6 | 46.3 | 39.9 |
| Non VdW | 17.0 | 18.0 | 15.2 | 17.5 |
| 1.4-VdW | 122.8 | 122.7 | 122.9 | 122.8 |
| Charge/Dipole | 37.8 | 37.9 | 37.7 | 37.8 |
| Dipole/Dipole | -20.4 | -20.4 | -20.4 | -20.4 |
| Total Energy | 265.3 | 265.2 | 266.5 | 265.2 |
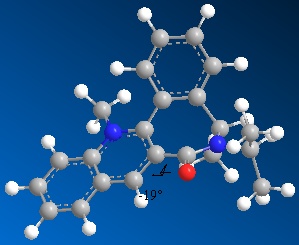
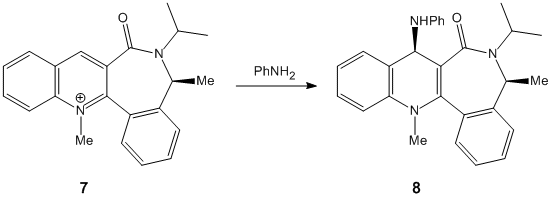
When this molecule reacts with an NHPhenyl reagent to from the product in (FIGURE ???). The stereochemistry of the product is best explained by discussing how the reaction takes place. Unlike in the previous example, the reagent has no ability to coordinate to the oxygen of the carbonyl group. The nitrogen cannot coordinate since it is neither electropositive nor small and unhindered as the Grignard magnesium reagent, and so the delivery of the NHPh group does not follow the same pattern as the delivery of the Me group in the previous example. Instead the selectivity of the reaction is ‘controlled by the sterics of the carbonyl’ CITE CITE CITE group.
As seen by the previous energy data for the MM2 calculations for the molecule, the conformer is more thermodynamically stable when the carbonyl group is orientated below the plane of the pyridine ring. The proximity of the carbonyl group and the NHPH group to be added on position 4 of the pyridine means that this new group will be sterically directed to the top face of the pyridine ring where there is less hindrance from the carbonyl group. In addition to this steric factor it should also be considered that the NHPh group will be directed away from the Carbonyl (i.e.to the top face of the aromatic ring) due to the fact that both the oxygen and nitrogen are electronegative and have lone pairs of electrons, that if forced into close proximity on the same plane of the ring would reduce the stability of the molecule though electron-electron repulsion.
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol ==
The following molecules were modelled Chemdraw 3D and MM2 calculations used to minimise the energy. The energies of the conformers were found to be significantly effected by the conformation of the 6 membered ring in the molecule. The six membered ring was found to be able to have a chair or a twist boat conformation.
When the models were drawn and the minimums calculated initally I found the 6 membered ring automatically assumed the chair conformation. For a cyclo hexane moleucle the chair is the most stable but I wanted to provide the data for the twist boat conformer in order to give evidence that the chair conformation of the 6 membered ring in the taxol molecule would give the lowest enrgy conformer. It was difficult to manipulate the molecule in order for the twist boat conformer to be adopted. The boat conformer is so energetically unfavourable that the MM2 calculations would not find it. Following is the energy data for the Taxol molecule.
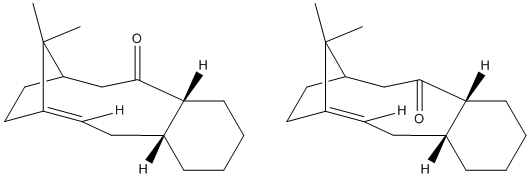
It is found that the molecule taxol is 'hyper stable', i.e. that its double bond reacts unexpectedly slowly. This is noticible by the fact that when the bond is hydrogenated the energy of the molecule actually increases. The reason for the hyperstability of the molecule is due to the additional strain changing the 2 carbons from sp2(double C-C bond) to sp3 puts on the molecule (seen as 'torsion' in the energy data). This additional torsion is a result of the proximity of the additional Hydrogen atoms added in hydrogenation. The trigonal planar conformatioon of the sp2 carbons has an angle of 120 whereas the hydrogenated tetrahedral sp3 carbons are at a 109 degree angle. In such a large ring there will be extra strain as a result of this reduction in angle.
== Modelling Using Semi-empirical Molecular Orbtial Theory ==
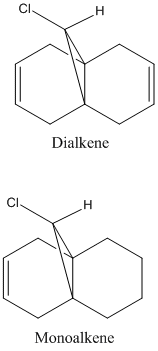
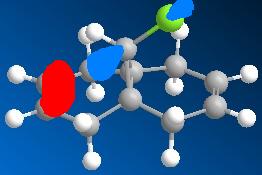
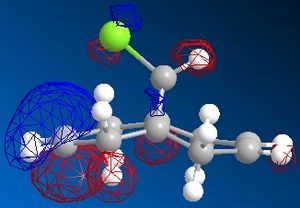
The dichlorocarbene molecule was modelled on Chem Draw 3D and the energy minimised by MM2 calculations. MOPAC/PM6 methods were then used to provide a representation of the molecular orbitals for the molecule. The HOMO (Highest Occupied Molecular Orbital) was of particular interest as it could give an idea of which of the 2 alkene functional groups were likely to be attacked by an electrophille. The representations show that the alkene on the same side as the Chlorine atom was where the HOMO was found, there was very little HOMO electron density on the other alkene in comparison. This shows that the alkene on the same side as the chlorine is most susceptible to electrophillic attack.
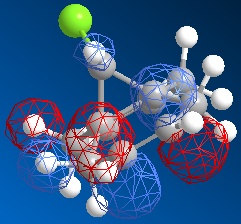
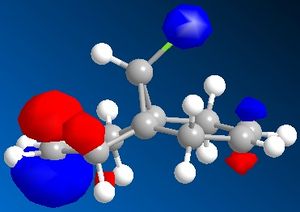
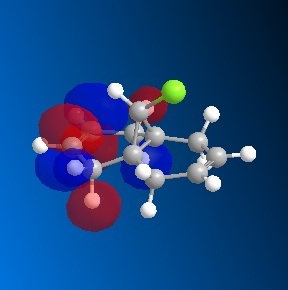
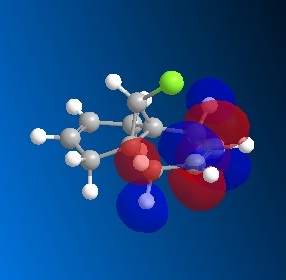
Gaussian Interface was then used in order to visualise the bending and stretching of the molecule and also of the same molecule with only one double bond). The wavenumbers of the stretches follow:
The difference in stretching frequencies for each of the molecules can be explained by the change in electron density. The higher the frequency of the stretch, the stronger the bond that is stretching. The data above shows that the mono-alkene has a stronger C-Cl bond than the dialkene. This is due to the interaction of the carbon-chlorine σ* orbital with the electron density of the alkene on the alternate side than the chlorine. This interaction means that there is some overlap of electron density into the anti bonding orbital of the C-Cl bond, this weakens the bond. In the mono-alkene, where this double electron density has been removed, there is less overlap into the C-Cl σ* orbital and so the C-Cl bond is stronger in this molecule.
