Organic:HughLaurie
The Hydrogenation of the Cyclopentadiene Dimer
Dimer Isomers
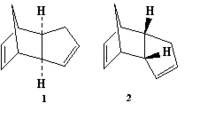
Cyclopentadiene molecules are known to undergo a dimerisation which gives rise to the endo dimer (labelled '2' in the picture to the right), as opposed to the exo dimer (labelled 1). With this in mind, the two isomers were modelled in ChemBio3D, and their energies were both minimised using the MM2 program. Their respective energies, when minimised, were as follows:
| Isomer | Energy (KJ/mol) |
|---|---|
| Exo | 133.4 |
| Endo | 142.3 |
From this data, it is apparent that although the endo isomer is the known product of the dimerisation, the exo isomer is the lower in energy. Therefore, as the favoured product of the reaction is not the thermodynamic product (i.e. that which is lower in energy), the dimerisation reaction must be under kinetic, rather than thermodynamic control.
Hydrogenation
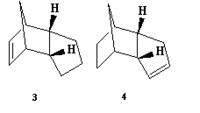
The ChemBio3D and MM2 programs were now applied to two possible products of the hydrogenation of endo dimer '2', here labelled as isomers '3' and '4'. Both were modelled, and their energies minimised, and various data was obtained concerning each molecule (all values in the table being in KJmol-1):
| Value | Molecule 3 | Molecule 4 |
|---|---|---|
| Stretch | 5.25 | 4.59 |
| Bend | 79.91 | 60.69 |
| Stretch-Bend | -3.48 | -2.30 |
| Torsion | 46.59 | 52.29 |
| Non-1,4 VDW | -6.89 | -4.40 |
| 1,4 VDW | 24.24 | 18.88 |
| Dipole Dipole | 0.68 | 0.59 |
| Total Energy (KJ/mol) | 146.30 | 130.35 |
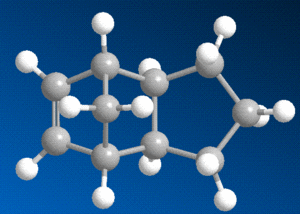
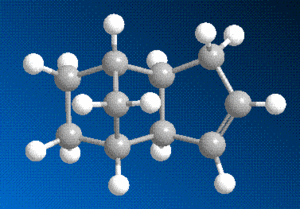
The data in this table reveals that molecule 4, with the double bond in the 5-membered ring as opposed to the 6-membered ring, is the lower in energy of the two, and will hence be the more thermodynamically stable. The difference in energy between the two isomers can be partially explained by using the relative values in the table above in conjunction with the 3D diagrams of each molecule.
The strain due to bending is the most significant factor in the evident energetic disparity - the breaking of the double bond provides some relief in this respect for both isomers, but substantially more so when it is the bond in the six-membered ring, this is due to the steric arrangement of said bond, close to the bridging group.
Perhaps surprisingly though, the torsional strain of molecule 4 is noticeably higher than in molecule 3. This can be explained by looking closely at the two diagrams - although the breaking of the bond in the six-membered ring lowered the bending strain, the subsequent addition of hydrogens in that region of the molecule will cause a repulsive interaction with the hydrogens on the adjacent carbon and with those on the bridging group, which causes the molecule to twist.
The chief consequence of the relative thermodynamic stability of molecule 4 is that, because the most common method of hydrogenation involves a catalytic reaction (e.g. Wilkinson catalysis[1]), the activation energy will be lowered, and the thermodynamic product - molecule 4 - will predominate.
 |
 |
Stereochemistry of Nucleophilic Additions to a Pyridinium Ring

Prolinol
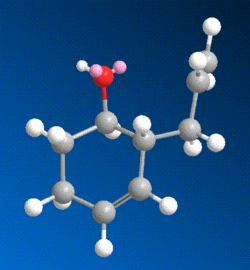
To the right is shown a stereospecific reaction involving the addition of a methyl group to a pyridinium ring, in this case that of prolinol. This stereospecificity (meaning the explicit addition of the methyl group to the 4 carbon of the pyridine ring, projecting out of the screen as observed in the diagram) can be explained by taking into account the mechanism of the reaction. When a molecule with a pyridine ring adjacent to a carbonyl group reacts with a Grignard reagent, it has been observed that the Magnesium atom of the Grignard coordinates to the carbonyl oxygen[2]. This then directs the nucleophilic methyl group towards the pyridine ring on the same side as that of the carbonyl group.
So, considering the effect that the geometry of the carbonyl group could have on the stereochemistry of this reaction, the prolinol molecule was modelled in ChemBio3D, and its energy was minimised. The resulting model clearly shows that, in the most thermodynamically stable form of prolinol, the carbonyl oxygen projects slightly above the plane of the aromatic pyridine ring, as shown below to the right, causing the methyl group to add to the ring on the same side - above the plane.
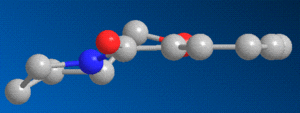
Addition of an NHPhenyl group to a pyridinium based reactant
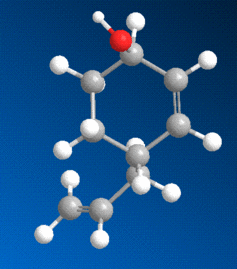
A similar analysis was now conducted with molecule 6, shown to the left. The reaction shown here gives a similarly stereospecific product to that of the prolinol reaction, with the added group again projecting outwards, but in this case the reactant, and the mechanism by which the product is obtained, are entirely different.
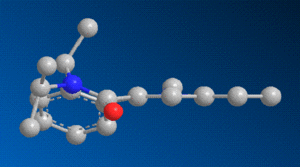
When modelling the initial reactant, molecule 6, in ChemBio3D, and minimising its energy, the carbonyl oxygen adjacent to the pyridine ring was this time found to be orientated slightly below the aromatic plane, in contrast to prolinol, in which it was above. The difference between the two can be seen by comparing the 3D models.
As previously stated, in the case of the grignard reaction, the added group was directed towards the same side of the plane as the carbonyl group. In this reaction, there is no Magnesium atom coordinating to the oxygen, and so the incoming amine group is directed towards the opposite side to the carbonyl by steric and coulombic interactions. First of all, the bulky Phenyl group being added to the molecule will favour a position as far away as possible from adjacent groups, and secondly, the lone pairs on the nitrogen of the amine will have a repulsive interaction with those of the oxygen atom. Taking this into account, and bearing in mind the position of the carbonyl group, it stands to reason that the addition reaction of a bulky amine group will be very stereospecific, and that the amine will always coordinate more favourably to the plane on the opposite side to the carbonyl.
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
Two atropisomers of an intermediate in the synthesis of taxol are shown to the right, one with the carbonyl group pointing up (A), and one with the group pointing down (B).

For the purpose of determining which of the structures was the most energetically stable, both of these structures were built and optimised using MM2 force field. Of course, with the large cyclic structure of the molecule, various different conformers are possible in the construction of this molecule, and so the molecules were constructed from several different starting points in order to find the most suitable conformer. In all conformers, the most stable structure appeared to be the 'down' isomer (isomer B as drawn in the diagram to the right).
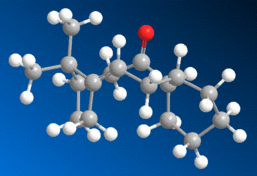
The most stable conformer (found to be a 'twist boat' conformation), in its 'up' and 'down' form, is shown below in 3D
 |
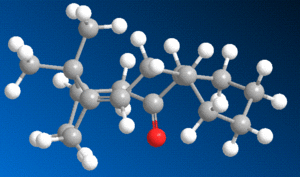 |
The energy of the up form was calculated at 227.6 KJmol-1, whereas the energy of the down isomer was found to be 211.7 KJmol-1, so it is quite apparent that the most stable isomer of this compound is that with the carbonyl group pointing down, as shown above to the right.
This molecule is an example of a 'hyperstable alkene', meaning that, due to the fact that the alkene bond is at the 'bridge head' (adjacent to the bridging group), the molecule is stabilised relative to its equivalent alkane form - this stabilisation is known as the 'olefin strain' (OS) energy[3] [4]. The source of the stabilisation is in the broader angle permitted around the strained bridging area by the presence of a double bond. Hydrogenating this bond thus has a destabilising effect on the molecule, explaining its tendency to react very slowly.
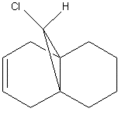
Regioselective Addition of Dichlorocarbene
Orbital Data of Bicyclic Diene

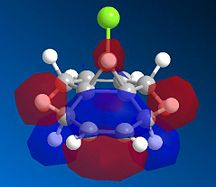
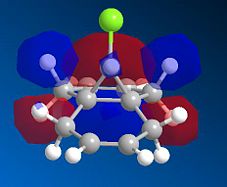
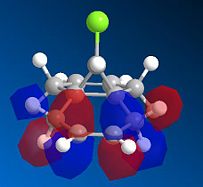
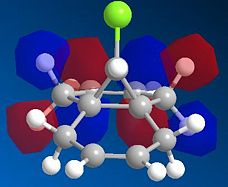
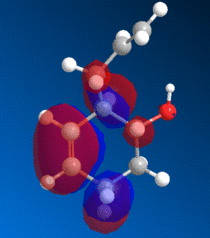
The bicyclic diene illustrated to the left was the subject of a more detailed computational analysis using the MOPAC/PM6 program to study the reactivity of the molecule in relation to its orbital properties.
First of all, the molecule was constructed in ChemBio, and its geometry was optimised using MM2 force field. The energy was then minimised further using the MOPAC program, and various molecular orbitals could be observed. Several orbitals around the HOMO/LUMO region, as obtained by this method, are displayed below:
 |
 |
 |
 |
 |
Examining these orbitals, and specifically the HOMO, it becomes apparent that the electron density around the double bond at the chlorine's side of the molecule is greater than that at the other double bond. This suggests that the double bond endo to the chlorine will be more nucleophilic, and therefore more susceptible to electrophilic attack than its counterpart at the other end of the molecule. Clearly then, the MOPAC method demonstrates that there is a very clear distinction between the two double bonds.
IR Comparison of the Diene and its Partially Hydrogenated Derivative
Next, the MOPAC optimised diene compound, and a derivative compound in which the alkene bond exo to the chlorine was hydrogenated, both underwent a further geometry optimisation using the B3LYP/6-31G(d,p) Gaussian program, which also obtained their IR spectroscopy data. Observing the IR data in Gaussview, it was possible to show which peaks in the spectra corresponded to which molecular vibrations. The C-Cl and C=C bond stretch peaks for each molecule are given in the table below (obviously there is no exo C=C peak for the monoalkene, as this bond was hydrogenated):
| Peak | Diene | Monoalkene |
|---|---|---|
| C-Cl | 770.8 | 779.8 |
| Endo C=C | 1757.4 | 1753.8 |
| Exo C=C | 1737.0 | N/A |
What is striking about these results is that, with hydrogenation, the wavenumber of the C-Cl peak is seen to increase, meaning that there is a corresponding increase in the strength of the bond. This is presumably due to the destructive interaction of the exo π orbital with the σ* orbital of the C-Cl bond - when this interaction is removed, as it is when the exo double bond is hydrogenated, the C-Cl bond is shortened, and consequently becomes stronger.
In the case of the C=C bonds, the wavenumber actually decreases slightly, suggesting a weakening of the endo double bond with the breaking of the exo bond. This is possibly in response to the shortening of the C-Cl bond, meaning that the stabilising overlap of the endo π orbital with the other side of the σ* orbital is reduced.
The extremes of the discussed vibrations are illustrated below:
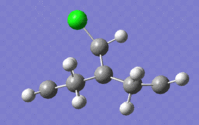 |
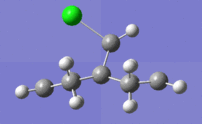 |
 |
 |
Structure Based Mini Project Using DFT-based Molecular Orbital Methods
Isomers and MOs

The reaction to the right is taken from a paper[5] published in 2000, relating to allylboration reactions. The specific reaction shown is stereoselective, and involves the opening of an epoxide ring and the trans addition of an allyl group to the molecule. As the paper says, the particular site at which the allyl group adds is dependent upon which metal the allyl group is coordinated to, and which solvent is used for the reaction - isomer 2 is most easily formed at low temperatures (-40°C), with a cuprate compound as the allyl-metal and THF as the solvent, whereas isomer 1 forms best at room temperature in diethyl ether with a Grignard reagent.
The two isomers were both modelled in ChemBio and their structures were optimised using MM2 force field and then MOPAC. The resulting models are shown below.
 |
 |
A point of interest is that Isomer 1 is the more thermodynamically stable, with an energy of 32.05 KJmol-1 , whereas Isomer 2 had the slightly higher energy of 35.0 KJmol-1. In a way, this is expected, as Isomer 1 forms more readily at higher temperatures. This is also supported by observing the molecules in terms of their MOs -
 |
 |
Looking at these models, it is clear that the HOMO of Isomer 1 is by far the more ordered, and that Isomer 2 suffers from destructive interference between orbitals, due to the proximity of the double bond to the hydroxy group and adjacent allyl chain. This supports the finding that isomer 1 is the more stable.
Spectroscopy
When attempting to distinguish between these two isomers, the most reliable technique to use would be NMR spectroscopy, either C-13 of H-1. The two isomers both underwent a Gaussian structure optimisation in order to obtain their C-13 NMR data.
References
- ↑ Donna J. Nelson, Ruibo Li, and Christopher Brammer J. Org. Chem., 2005, 70 (3), pp 761–767
- ↑ A. G. Shultz, L. Flood and J. P. Springer, J. Org. Chemistry, 1986, 51, 838.
- ↑ Wilhelm F. Maier, Paul Von Rague Schleyer J. Am. Chem. Soc., 1981, 103 (8), pp 1891–1900
- ↑ Alan B. McEwen, Paul v. R. Schleyer J. Am. Chem. Soc., 1986, 108 (14), pp 3951–3960
- ↑ Marek Zaidlewicz* and Marek P. KrzemińskiOrg. Lett., 2000, 2 (24), pp 3897–3899
