Org:pswallow89
The basic techniques of molecular mechanics and semi-empirical molecular orbital methods for structural and spectroscopic Evaluations
Aims
Computational methods will be used to rationalise the outcome of several reactions including the hydrogenation of a cyclopentadiene dimer, Nucleophilic additions to a pyridnium ring, the reactivity of a taxol intermediate and the regioselective addition of Dichloro-carbene. Following this using the techniques shown, a project investigating the regioselectivity of n3 co-ordianted allypalladium complexes will be undertaken.
Part 1: Modelling using Molecular Mechanics Hydrogenation of a Cyclopentadiene Dimer
Due to it's high reactivity cyclopentadiene will undergo a facile [4+2] cyclo-addition to give the possibility of forming both and exo dimer (fig.1) and an endo dimer (fig.2) It has been reported that the endo dimer is the exclusive product of the reaction and the reasons for which can be shown using the energies of the products:
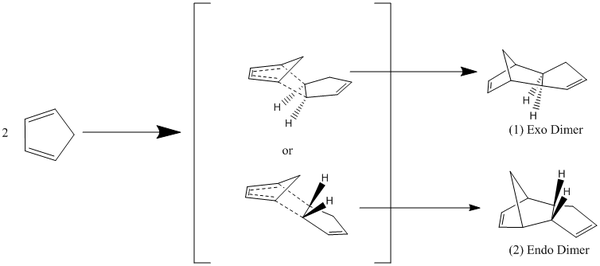
The relative energies of the 2 products determined by minimising the energy using a MM2 forcefield were 31.88kcal/mol for the exo dimer and 34.00kcal/mol for the endo dimer. The thermodynamic product of the reaction would be the exo dimer as it is the structure lowest in energy and therefore, theoretically the most stable. However, as it is the endo product that is formed exclusively it would suggest that the reaction is under kinetic control rather than thermodynamic control. The Exo dimer is the kinetic product in accordance with the endo rule of the endo product having the transition state, which is lowest in energy and thus being the kinetic product [1]. The basis of a more stable transition state must come from stereo-electronic means as the endo transition state is sterically disfavoured through the clash between the hydrogens and bridging methylene group. Hence the stabilisation arises from the HOMO-LUMO interactions in the two transition states :
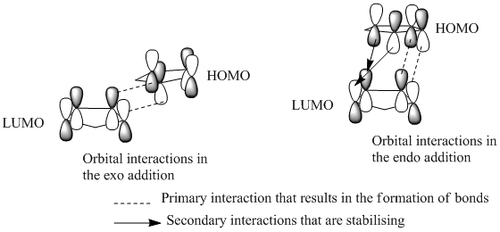
It can be seen that the endo transition state has additional stabilising interactions between the orbitals that are not-involved in the bonding and the stabilisation from these interactions is the reason for the endo product being favoured so much.The role of these orbitals is highlighted in by replacing cyclopentadiene with cyclopentene, a reaction which gives only 29% of the endo product even when reacted for longer times at higher temperatures [1]
On Hydrogenation of the endo product with one equivalence of Hydrogen gives the possibility of forming the two following products:

The two products were evaluated using a MM2 forcefield to determine several of their energetic properties, which are summarised in the following table:
| Properties | Hydrogenation product 1 | Hydrogenation product 2 |
|---|---|---|
| Stretch | 1.30 | 1.11 |
| Bend | 19.82 | 14.49 |
| Torsion | 10.82 | 12.50 |
| Non-1,4 Van Der Waal | -1.20 | -1.03 |
| Van Der Waals (VDW) | 5.63 | 4.50 |
| Dipole-Dipole | 0.162 | 0.147 |
| Total Energy (kcal/mol) | 35.67 | 31.17 |
The product formed will depend on the reaction conditions. If the reaction is under thermodynamic conditions where the most stable product is formed, from the total energies it can be seen that the 2nd possible Hydrogenation product is more likely to be formed that Hydrogenation Product 1 as it is predicted to be more stable by 3.5kcal/mol. However, if the reaction is under kinetic conditions where the product with the most stable transition state is the product formed. This is likely to hydrogenation product 1 as it has the larger steric strain by 5.33kcal/mol. The strain can also be seen by considering the angles of the alkene that is hydrogenated which are 107° compared to 113° for the C=C which when Hydrogenated leads to the second of the Hydrogenated products. Given that the idealised angle for a sp2 carbon is 120o it can be seen that the more highly strained alkene will be the more reactive and so is most likely to react faster. This will especially be a factor as the Hydrogenation is likely to be metal catalysed by either Ni or Pd based systems which favour the release of ring strain for the driving force of the reaction. Consequently, given that the "Hydrogenation product 2" is formed exclusively the reaction must be under kinetic control.
Key Literature
- M Fox, R Dardona, N Kiweit J. Org Chem 1987, 1469
Stereochemistry of Nucelophilic addition to pyridnium ring
It has been reported in Literature that Grignard addition to a Pyridinium ring adjacent to a Carbonyl shows surprising selectivity at the carbon beta to the Carbonyl group. The following reactions between molecules (5 and 7) were investigated using molecular mechanics methods to determine the minimum energy states of the reactants and use the analysis as a rational for the product formed. The addition of a Grignard to the Pyridinium ring is suspected to proceed via co-ordination between the lone pairs on the oxygen and Magnesium providing the stereochemical control observed:

The evident co-ordination demonstrated above means that the relative position of the oxygen relative to pyridinium ring in the most stable product will be able to control the stereochemistry of the product. The had an energy of 44.6kcal/mol obtained and this corresponded to a dihedral angle of 11.4o. Given that the dihedral angle is positive in the lowest energy state, the carbonyl group must be pointing above the plane of the ring. The result of which is the Magnesium and consequently the rest of the Grignard being held above the ring leading to the reaction occurring on the top face of the ring and the observed selectivity in the product. The interaction between Mg and O is enough to overcome the increased sterics that would be observed in the Transition state. However, in the attack of PhNH2 on molecule 7 we would expect sterics to dominate the stereo-chemistry as there is no way for the oxygen to co-ordinate with the incoming nucleophile. However, this cannot be modelled using the MM2 methods as the Mg atom is not in the basis set used for the calculations. If the Mg were to be modelled a different method using a different basis set should be used.
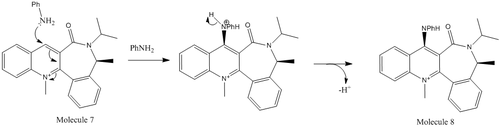
was minimised to 62.65kcal/mol using the MM2 method, for which the dihedral angle betweewn the carbonyl and pyridinium ring was found to be -17.8 degrees. The fact that the angle is negative indicates that the Carbonyl group is orientated to be pointing below the plane of the Pyridinium ring, which is presumably to minimise the lone-pair repulsion with the lone pair on N. The Oxygen now acts as a steric barrier to the incoming N based nucleophile's attack on the bottom face of the ring, which is what leads to the selectivity of addition to the top of the ring.
Key Literature
A. G. Shultz, L. Flood and J. P. Springer, J. Org. Chemistry, 1986, 51, 838. DOI:10.1021/jo00356a016
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
During the synthesis of Taxol one of the key intermediates can form a pair of atropisomers (9) and (10) which the carbonyl group can be above or below the plane of the ring. It has been reported that one of these forms in the majority. The origins in the difference of energies of the two isomeris arises from the synthetic route taken, which can form the cyclohexane ring in varying different conformations. An MM2 and MMFF94 minimisation of the molecules was carried out to determine which atropisomer is the most stable:
| Properties | Minimum energy MM2 Force Field | Minimum energy MMFF94 Force field |
|---|---|---|
| Molecule 9 | 50.59kcal/mol | 77.90kcal/mol |
| Molecule 10* | 41.8kcal/mol | 60.63kcal/mol |
- Originally the values obtained were 48.79kcal/mol and 69.272kcal/mol for the MM2 and MMFF94 force fields respectively. However, it was noticed that varying the conformation of the cyclohexane substituent adjacent to the carbonyl between the chair, boat and twist-boat conformations allowed a much lower energy to be obtained which is presumably a consequence of lowering the steric repulsion between the adjacent methylene group and the oxygen lone pair.
Molecule 9
High energy taxol |
Molecule 10
Mol 10 |
It had been shown that molecule 10 ie. Where the carbonyl group is pointing down is the most stable and is therefore, likely to be the most prominent isomer. This is going to be due to the decreased steric repulsion between the bridging methylene group and the lone pair’s on the oxygen. It can be seen that whilst the MM2 and MMFF94 methods give different energies, which is due to the differing methods used in calculation of the energy but the relative difference between the two isomers is similar.
The alkene is very stable, often termed hyperstable, due to the stability of the alkene relative to the parent alkane, which is given by the Olefin Strain Energy. Running an MM2 calculation on the theoretical product of the hydrogenation an energy of 56.14kcal/mol was obtained an OSE of 15kcal/mol. The result of this is a very positive Gibbs Free energy for the reaction and large strain (shown by the increase in the torsional and bending energies in the alkane) in the new sp3 hybridised carbon in the product compared to the sp2 hybridised Carbon. The reasons for such a disfavoured hydrogenation are due to the stability of the conformation of the ring in the starting material and the fact that the alkene being at a bridgehead carbon meaning that on hydrogenation the H atoms will be greatly disfavoured
Key Literature
- S. W. Elmore and L. Paquette, Tetrahedron Letters, 1991, 319; DOI:10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0
- See J. G. Vinter and H. M. R. Hoffman, J. Am. Chem. Soc., 1974, 96, 5466 (DOI:10.1021/ja00824a025
Part 2: Modelling using semi-empirical Molecular Orbital Theory
Regioselective addition of dichlorocarbene
The addition of a Dichlorocarbene to molecule 12, occurs by a form of a selective electrophilic attack on only the endo alkene with respect to the C-Cl bridging unit (although there is a side product given by the attack of a second dichlorocarbene molecule):

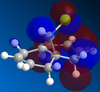
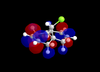
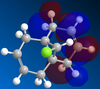
Given that the steric barriers for addition to the bottom of the ring are more or less equal for the endo (C=C on the left of the molecule) and exo (C=C on the right of the molecule) is therefore more likely to be found under orbital control and so the molecules must stable conformation was found using MM2 methods and then the MOPAC interface was used to produce the MOs for the complex.
 |
 |
 |
 |
|---|
It can be seen in the HOMO that there is significant overlap between the exo alkene's pi system and the σ*(C-Cl) anti-bonding orbital, which are anti peri-planar to each other. This an overall stabilising interaction, lowering the energy of the pi electrons making and thus making the exo alkene much less reactive compared to the endo alkene, accounting for the selectivity shown in the reaction. The effect of the orbitals is demonstrated by the decreased distance (which demonstrates and increased orbital interaction) between the exo-alkene and bridgehead group with the interaction compared to the endo alkene-bridgehead group distance without the interaction(2.546A and 2.565A respectively, from the MM2 calculations) To gain an insight into the magnitude of the difference in bond strengths brought about by the interaction the vibration spectra were reproduced for Molecule 12 and several of it's derivatives, which were di-substituted across the exo-alkene and the results obtained were summarised:
| Substituent on the exo alkene | Energy/kcal | C-Cl stetch/cm^-1 | Anti alkene stretch/cm^-1 | Syn Alkene stretch/cm^-1 |
|---|---|---|---|---|
| H | 17.91 | 770.9 | 1737 | 1757 |
| Me | 17.19 | 768 | 1747 | 1755 |
| OH | 10.72 | 781 | 1797 | 1757 |
| CN | 19.67 | 772 | 1670 | 1757 |
| BH2 | 16.9 | 761 | 1604 | 1757 |
| SiH3 | 18.69 | 767 | 1633.78 | 1755 |
| Mono alkene | 18.69 | 779.8 | N/A | 1754 |
From the data obtained it can be seen that going from H to Me gives a stabilisation of both the overall molecule and the Anti C=C bond (demonstrated by a higher frequency for the bond yet weakening the C-Cl bond. This is probbly due to the fact that the C=C is higher in energy and thus gives a larger overall stabilisation in when donating in the σ*(C-Cl) orbital in accordance with the Klopman-Salen equation (which shows the stabilisation energy is inversely proportional to the energy difference between the orbitals). Donation into the anti-bonding orbital weakens the C-CL and the anti-C=C bond as electron density moves away from the respective atoms and hence gives them a lower frequency. This destabilisation of the C-Cl is maxmised for SiH3 and BH2, both of which are highly electron donating groups and so can increase the energy of the C=C bond the most. CN substituents also show this effect but to a lesser extent due to the electronegativity of the group meaning the C=C orbital will contract slightly and hence not give as good overlap with the C-Cl σ* orbital. When OH is used as a substituent there is an actual increase in the frequencies of the C-Cl and anti-C=C bonds showing that they have been strengthened by the replacement of the H with OH.
Key Literature
- B. Halton, R. Boese and H. S. Rzepa., J. Chem. Soc., Perkin Trans 2, 1992, 447. DOI:10.1039/P29920000447
--- Mini Project Investigation into the unusual regio-selectivity of nucleophilic addition to an η3-allylpalladium complex
A literature report was found to report that on attack of 3-hydroxycoumarins (A) on η3-allylpalladium complex showed a high regio-selectivity. The following report will detail a investigation into whether the literature assignments are in agreement with calculations and if there was any reason for the selectivities shown. Overall Reaction Scheme:
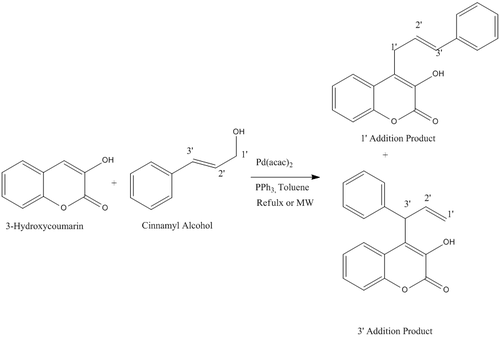
The mechanism for the reaction can proceed via one of two routes, nucleophilic attack on a n3 co-ordinated ally complex (mechanism 1) or direct nuckeophilic attack on the cinnamyl alcohol(mechanism 2)


For both of the addition products there was the possibility of several rotamers that could be used as starting points for the energy minimisations, each giving a different minima. Therefore, the energetics of the rotation about the newly formed C-C bond was evaluated using the dihedral driver/minimisation application in Chembio3D. The resulting graph gave a conformation with the angle between the cinnamyl and hydroxycoumarin groups 75o and the energy minimised to 7.92kcal/mol. This will therefore be the starting point of any further calculations of this molecule. A similar process was carried out on the 3' addition product which gave the most stable rotamer of the complex containing a dihedral angle of -80o and energy of 12.56kcal/mol. This will therefore, also be the starting point of the later calculations on this molecules. From these two starting points the energy was minimised by using both molecular mechanics (MM2) and MOPAC-PM6 methods to obtain the most stable structure relative to the positions. Following this the molecules had a final minimisation using Gaussian to give the most stable structure of the products. The 13C and 1H NMR spectra of both products was then calculated from this structure again using gaussianm and the values obtained will be compared to evaluate the accuracy of the assignments in the report The results turned are summarised in the following tables:
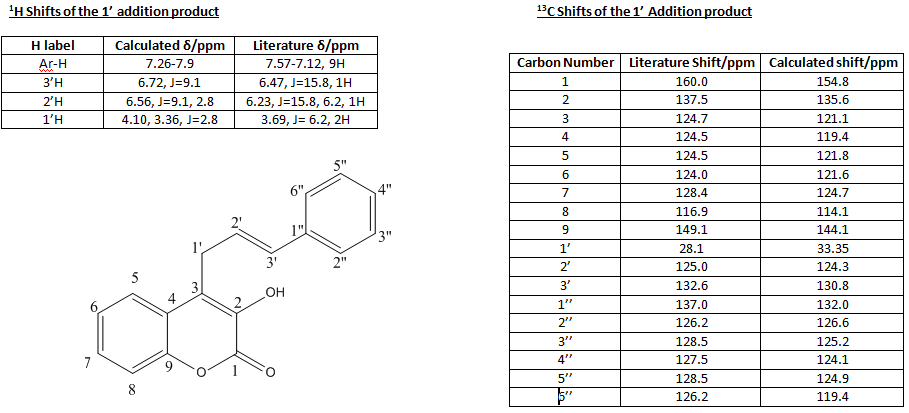
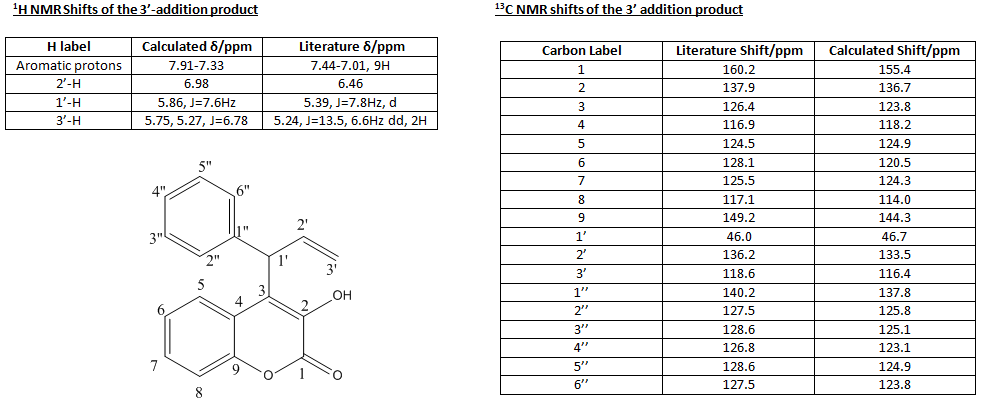
The shift values for the literature report have been correct as it was found that there was an error in the labelling of the Carbon and the relative shift for example C-2 in the report is actually C-1 (the carbonyl Carbon) and can be identified by the extreme de-shielding given by the two oxygen substituents. Once this error had been corrected for the shift values matched up reasonably well and can be shown by looking at the standard deviations between the calculated and reported values, which were 3.75ppm for the 1' addition product and 3.25 for the 3' addition product. The most important values concern the carbons denoted 1', 2' and 3' as it is through the values of these that the product can be identified. In the addition at the carbon labelled 1' in the starting reagent, the most de-shielded carbon (now labelled 1') forms a methylene group whereas when the reaction occurs at the 3' carbon in the starting reagent the product's 1' carbon contains is tertiary with a phenyl group having replaced one of the Hydrogens. As the phenyl group has a slight electron withdrawing effect through inductive methods compared to hydrogen we would expect the shift peak to be higher in the 3' addition product compared to the 1' addition product and this is what is replicated in both the experimental and calculated shift values. Similarly the 3' carbon in the product has a phenyl substituent in the 1'-addition and only 2 H substituents in the 3' addition and so the resonance will be found at a lower ppm in the 1' addition compared to the 3' addition and again this is seen in both of the experimental and calculated shift values. However, there was a discrepancy between the calculated coupling constants as they tended to be of a lower magnitude than the experimentally reported ones which is due to the less accurate calculations done to generate the 1H NMR giving a higher error value that is translated through to the coupling constants Therefore, it can be concluded that the identification of the major product being the 1' addition in the literature is correct.
Unfortunately there was no IR reported in the literature which could also be compared, IR would have been a useful tool in identification of the two regio-isomers as the C=C bond would have different stretching frequencies and thus would be able to differentiate between the two. However, given that these frequencies were not reported in literature there was no point in calculating the spectra as there is no reference point to check the accuracy of the calculations. Another useful piece of information to have been provided in the literature would have been the optical rotation of the 3' addition where the 1' Carbon is in fact a chiral centre and so any optical activity or lack of it would have evidence for the correct identification of the regio-isomer.
The fact that the 1' addition product is the major product gives evidence to several things. Firstly the rate of co-ordination to the metal centre is much faster than the rate of nucleophillic attack by on the cinnamyl alcohol. Secondly the 1' addition as the major product supports the theory that the product is formed by nuceleophillic attack by the 3-hydroxycoumarin on the co-ordinted complex as this would be favoured by the attack on the less sterically hindered carbon giving the 1' selectivity. The other possibility in the mechanism would be an insertion of the hydroxycoumarin into the bound allyl complex but this is likely to give a more equal proportion of the 2 regio-isomers as steric effect the insertion will be under statistical control more than steric control. The final point on the reaction mechanism that can inferred is that it may also be under a form of thermodynamic control. The 1' product is the major product as a more stable double bond being formed in accordance with Zaitzev's rule that a more highly substituted double bond is more stable.
In conclusion in the mini project the 13C and 1H NMR spectra were both successfully calculated for both of regio-isomers and showed good accordance with the literature values that were reported, supporting the assignments in the paper. However, the report also highlighted a mistake with the numbering in the table reporting the 13C NMR shifts. Further work that could be done would involve investigating the report into that substituting the OH group on the cinnamyl alcohol for an acetate group gave rise to the inverse selectivity with the 3' addition product being favoured. Therefore, calculations could be used to investigate the proposed mechanisms and the relative transition states to determine why there is this selectivity.
Key Literature
A. Mitra et Al, ARKIVOC 2003, 6, 96-103
Spectra Links
1' addition product http://hdl.handle.net/10042/to-6468
3' addition product http://hdl.handle.net/10042/to-6476
