Oana:compinorganic
Inorganic Computational Report: Oana Popescu
Day 1
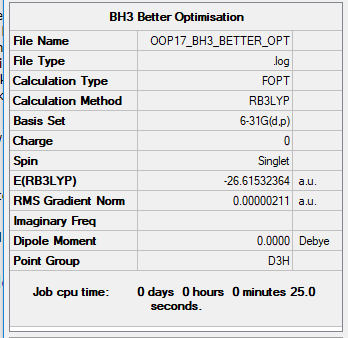
BH3
B3LYP/6-31B level
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000003 0.000300 YES Maximum Displacement 0.000017 0.001800 YES RMS Displacement 0.000011 0.001200 YES
Frequency analysis log file File:OOP17 BH3 FREQ.LOG
Low frequencies --- -1.1800 -1.0028 -0.0054 4.1927 11.0182 11.0637 Low frequencies --- 1162.9912 1213.1792 1213.1819
Optimised BH3 Molecule |
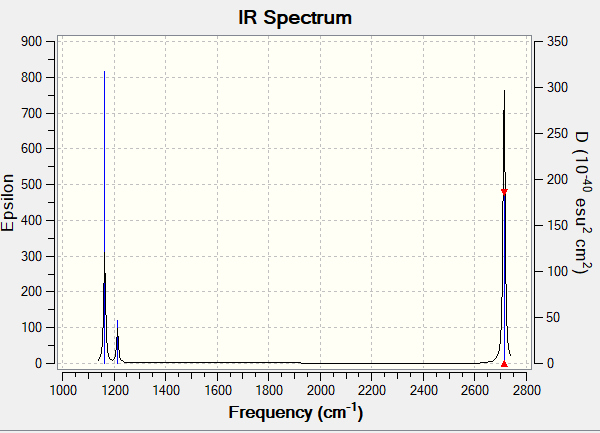
Vibrational spectrum for BH3
| wavenumber (cm-1) | Intensity (au) | symmetry | IR active? | Vibration Type |
| 1163 | 93 | A2" | yes | out-of-plane bend |
| 1213 | 14 | E' | very slight | asymmetric bend |
| 1213 | 14 | E' | very slight | asymmetric bend |
| 2582 | 0 | A1' | no | symmetric stretch |
| 2715 | 126 | E' | yes | asymmetric stretch |
| 2715 | 126 | E' | yes | asymmetric stretch |
Ng611 (talk) 18:14, 8 May 2019 (BST) Are the asymmetric bends out of plane or in plane?
Why does the IR spectrum have less than 6 peaks, even though there are 6 vibrations?
For vibrations to appear on an IR spectrum, they have to be IR-active, that is they should not be fully symmetrical (in which case they will cancel out and not produce any signal). Vibration 4 is a symmetric stretch and so does not appear on the spectrum; furthermore, vibrations 2 and 3 as well as 5 and 6 share the same wavenumber (as they are very similar) and so overlap on the spectrum, appearing as only one peak. The results is only 3 peaks being visible on the spectrum.
Ng611 (talk) 18:16, 8 May 2019 (BST) Good, remember that vibrations that share the same wavenumber and are similar are termed 'degenerate'
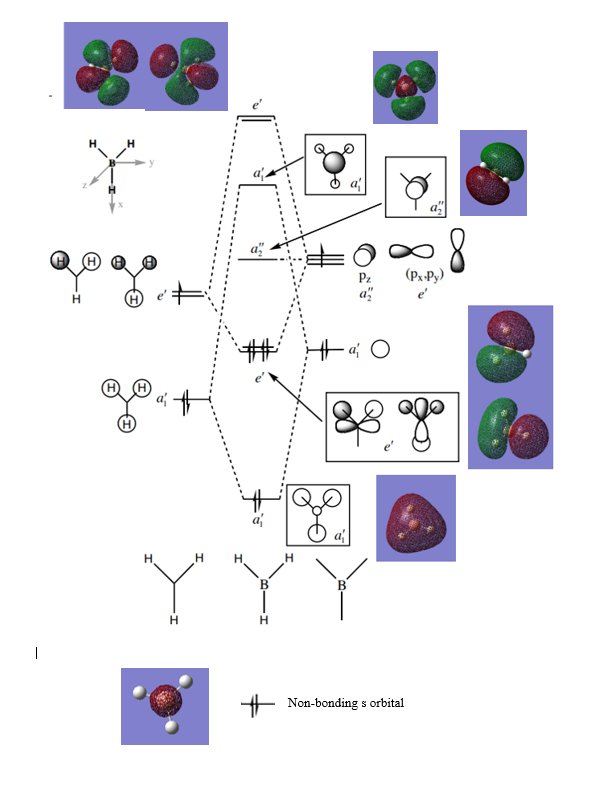
Ng611 (talk) 18:18, 8 May 2019 (BST) Your qualitative MO diagrams for your e' orbitals are missing here...
Are there any significant differences between the real and LCAO MOs?
Not any highly significant differences; they look generally the same. The most significant difference would be the a1' MO, which is much more spread in the real MO rather than the LCAO; however, this is something that can be assumed.
What does this say about the accuracy and usefulness of qualitative MO theory?
MO theory is an accurate way to approximate LCAOs appropriately and thus a highly useful tool from an inorganic chemistry perspective.
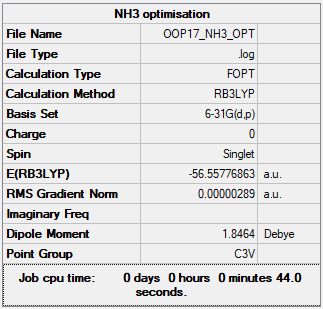
NH3
B3LYP/6-31B level
Item Value Threshold Converged? Maximum Force 0.000005 0.000450 YES RMS Force 0.000003 0.000300 YES Maximum Displacement 0.000010 0.001800 YES RMS Displacement 0.000007 0.001200 YES
Frequency analysis log file File:OOP17 NH3 FREQ.LOG
Low frequencies --- -11.4825 -11.4467 -0.0034 0.0247 0.1420 25.6342 Low frequencies --- 1089.6621 1694.1736 1694.1740
Optimised NH3 Molecule |
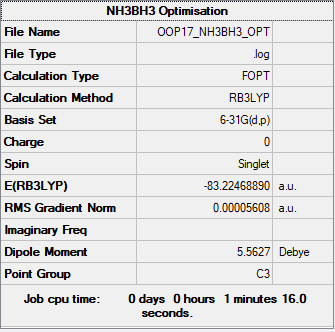
NH3BH3
B3LYP/6-31B level
Item Value Threshold Converged?
Maximum Force 0.000164 0.000450 YES
RMS Force 0.000035 0.000300 YES
Maximum Displacement 0.000903 0.001800 YES
RMS Displacement 0.000344 0.001200 YES
Frequency analysis log file File:OOP17 NH3BH3 FREQ.LOG
Low frequencies --- -27.5507 -0.3246 -0.0293 0.0365 12.1519 12.2518 Low frequencies --- 261.3987 631.2505 637.5548
Optimised NH3BH3 Molecule |
Bond energy = -83.2247 - (-26.6153 + -56.5578) = -0.0516 au = -135kJ/mol
Is the B-N dative bond weak, medium or strong?
In comparison to a C-H bond, which is around 413kJ/mol [2], this bond can be considered weak.
Ng611 (talk) 18:21, 8 May 2019 (BST) Good calculation, and good comparison, well done.
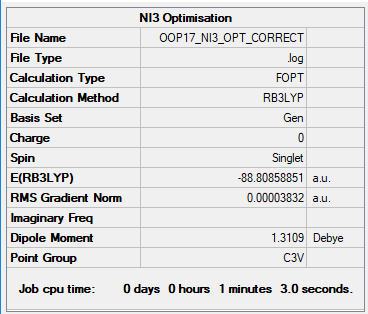
NI3
B3LYP/6-31B level
Item Value Threshold Converged? Maximum Force 0.000067 0.000450 YES RMS Force 0.000044 0.000300 YES Maximum Displacement 0.000492 0.001800 YES RMS Displacement 0.000333 0.001200 YES
Frequency Analysis Log File: File:OOP17 NI3 FREQ CORRECT.LOG
Low frequencies --- -12.7375 -12.7314 -6.2899 -0.0039 0.0188 0.0633 Low frequencies --- 101.0325 101.0332 147.4122
Optimised NI3 Molecule |
Days 2-3 Project: Ionic Liquids
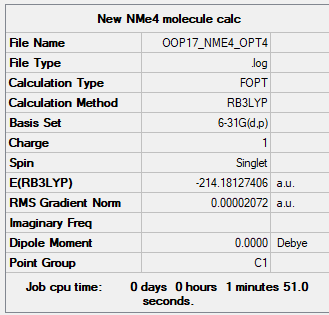
[N(CH3)4]+
B3LYP/6-31B level
Item Value Threshold Converged? Maximum Force 0.000069 0.000450 YES RMS Force 0.000017 0.000300 YES Maximum Displacement 0.001258 0.001800 YES RMS Displacement 0.000420 0.001200 YES
Frequency Analysis Log File: File:OOP17 NME4 FREQ2.LOG
Low frequencies --- -20.3323 -17.4320 -9.2161 -0.0003 0.0004 0.0008 Low frequencies --- 181.1492 284.0668 286.6779
Optimised [N(CH3)4]+ Molecule |
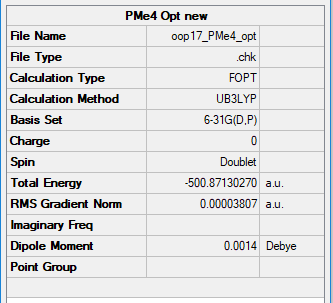
[P(CH3)4]+
B3LYP/6-31B level
Item Value Threshold Converged? Maximum Force 0.000130 0.000450 YES RMS Force 0.000039 0.000300 YES Maximum Displacement 0.001147 0.001800 YES RMS Displacement 0.000336 0.001200 YES
Frequency Analysis log File: File:OOP17 PCH34+ FREQ.LOG
Low frequencies --- -0.0016 0.0014 0.0016 50.2331 50.2331 50.2331 Low frequencies --- 185.7653 210.7918 210.7918
Ng611 (talk) 18:26, 8 May 2019 (BST) Looks like something went wrong with your calculation here as both your energy and normal modes are off.
Optimised [P(CH3)4]+ Molecule |
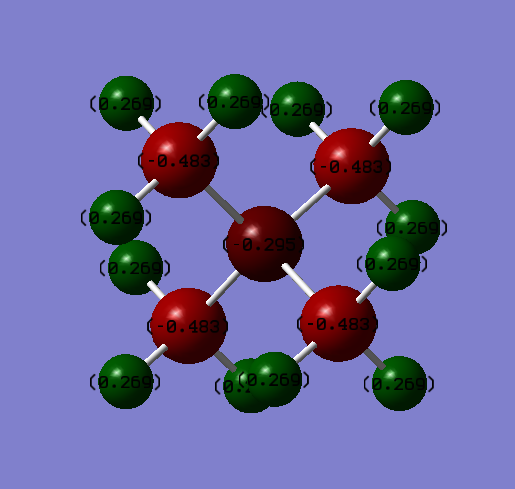
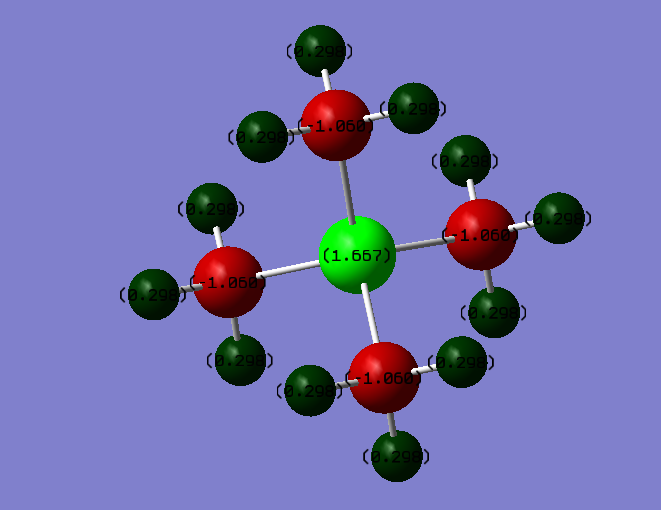
Charge Comparison
The charge distribution on an [N(CH3)4]+ ion.
| Atom | Charge |
|---|---|
| Hydrogen | 0.298 |
| Carbon | -0.483 |
| Nitrogen | -0.295 |
The charge distribution on a [P(CH3)4]+ ion.
| Atom | Charge |
|---|---|
| Hydrogen | 0.298 |
| Carbon | -1.060 |
| Phosphorus | 1.667 |
The Charge on the H-atoms is 0.298 in both cases; this remains unchanged, as the C-H electronegativity difference stays constant. In the Nitrogren ion, the central atom has a charge of -0.295, with the carbon atoms being -0.483 each. In the Phosphorus ion, the central atom has a charge of 1.667, with the carbon atoms being -1.060 each. The reason for this difference is electronegativities; Nitrogen is a lot more electronegative than carbon, so it retains a negative charge, with the carbon atoms being a lot less negative. Meanwhile, Phosphorus is more elecropositive than carbon, retaining a high positive charge with the carbon atoms having a much higher negative charge. Thus, the Carbon-Phosphorus bond has a much higher ionic characteristic that the Carbon-Nitrogen bond.
Ng611 (talk) 18:31, 8 May 2019 (BST) Good description of electronegativities, but none discussion of symmetry, why not?
What does the "formal" positive charge on the N represent in the traditional picture? The formal charge represents the overall charge of the molecule assuming the charge is spread equally among the atoms. In the traditional sense, the ionic plus is placed on the central atom to represent the entire molecule being formally positive, but converging on the nitrogen.
Ng611 (talk) 18:31, 8 May 2019 (BST) This description is a little unclear and vague and could have used some more detail. What do you mean by 'converging on the nitrogen'