Nt814
Part 1: Revision and Introduction
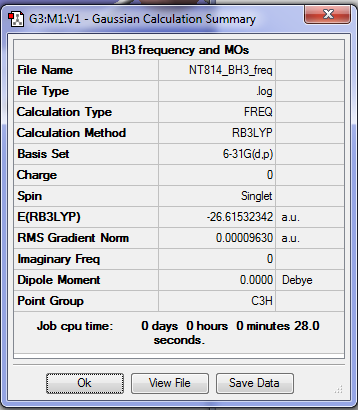
BH3
Optimisation
The method used for the optimisation was B3LYP using the basis set 6-31G. A Summary Table produced by Gaussview and the Item Table from the optimisation .log file can be seen below:
Item Value Threshold Converged? Maximum Force 0.000193 0.000450 YES RMS Force 0.000096 0.000300 YES Maximum Displacement 0.000759 0.001800 YES RMS Displacement 0.000379 0.001200 YES
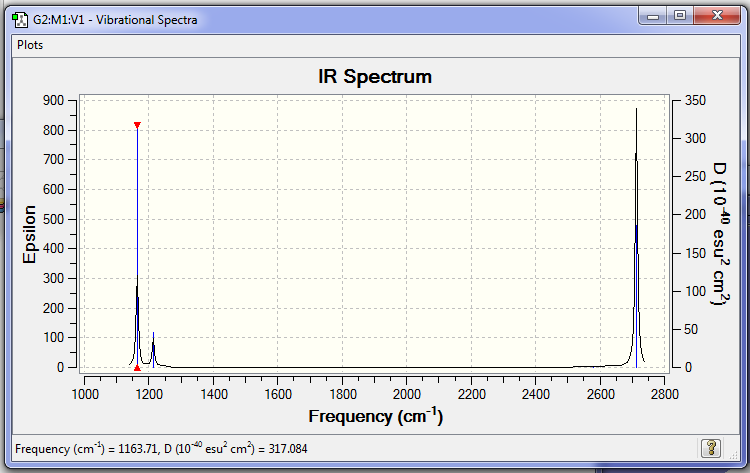
Frequency Analysis
Frequency analysis .log file: File:NT814 BH3 631G D3H OPT.LOG
The low frequencies for the optimisation were:
Low frequencies --- -0.1081 -0.0046 0.0009 46.4026 46.4028 47.3972 Low frequencies --- 1163.7065 1213.6310 1213.6312
Jmol Dynamic Image
BH3 |
Molecular Vibrations
| wavenumber (cm-1) | Intensity (arbitrary units) | symmetry | IR active? | type |
|---|---|---|---|---|
| 1163 | 92 | A2" | Yes | Out of plane bending |
| 1213 | 14 | E' | Slightly | Asymmetric bend |
| 1213 | 14 | E' | Slightly | Symmetric bend |
| 2580 | 0 | A1' | No | Symmetric stretch |
| 2713 | 126 | E' | Yes | Asymmetric stretch |
| 2713 | 126 | E' | Yes | Asymmetric stretch |
As there are two pairs of degenerate vibrations and one of the vibrations is IR silent, fewer vibrational peaks are observed than the number of vibrations.
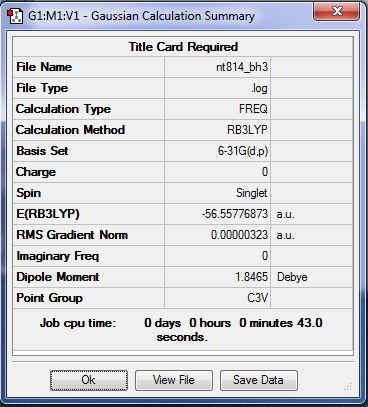
NH3
Optimisation
As above, the method used for the optimisation was B3LYP using the basis set 6-31G. A Summary Table produced by Gaussview and the Item Table from the optimisation .log file can be seen below:
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000014 0.001800 YES RMS Displacement 0.000009 0.001200 YES
Frequency Analysis
Frequency analysis .log file: File:NT814 NH3 OPT FREQ.LOG
The low frequencies for the optimisation were:
Low frequencies --- -0.0138 -0.0032 -0.0015 7.0783 8.0932 8.0937 Low frequencies --- 1089.3840 1693.9368 1693.9368
Jmol Dynamic Image
NH3 |
Molecular Vibrations
| wavenumber (cm-1) | Intensity (arbitrary units) | symmetry | IR active? | type |
|---|---|---|---|---|
| 1089 | 145 | A1 | Yes | Out of plane bending |
| 1694 | 14 | E | Slightly | Asymmetric bend |
| 1694 | 14 | E | Slightly | Symmetric bend |
| 3461 | 1 | A1 | Slightly | Symmetric stretch |
| 3590 | 0 | E | No | Asymmetric stretch |
| 3590 | 0 | E | No | Asymmetric stretch |
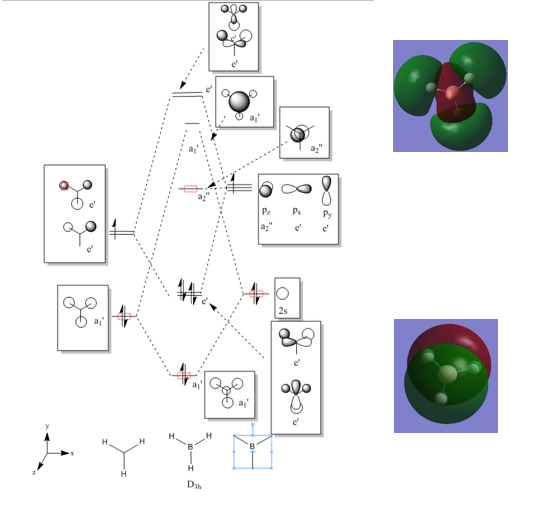
MO Diagram
Below an MO diagram for BH3 can be seen with the real HOMO and LUMO.
Ng611 (talk) 22:12, 15 May 2018 (BST) Why just the HOMO/LUMO? Why not all of the orbitals?
NH3BH3
Optimisation
An optimisation of the NH3BH3 molecule was carried out(6-31G/B3LYP), a results summary and item table for which can be seen be seen below.
Item Value Threshold Converged? Maximum Force 0.000115 0.000450 YES RMS Force 0.000060 0.000300 YES Maximum Displacement 0.000582 0.001800 YES RMS Displacement 0.000346 0.001200 YES
Frequency Analysis
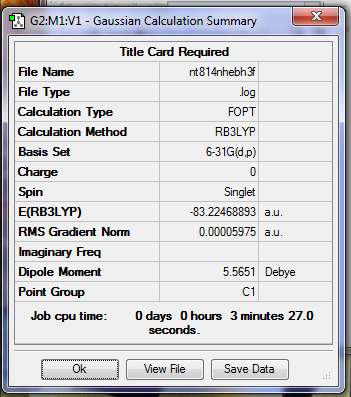
frequency analysis .log file: File:NT814NHEBH3F.LOG
Low frequencies --- 0.0005 0.0007 0.0009 17.2557 18.1773 37.5151 Low frequencies --- 265.8745 632.2229 639.3702
Jmol Dynamic Image
NH3BH3 |
BBr3
Optimisation
An optimisation of the BBr3 molecule was carried out (6-31G/B3LYP), a results summary and item table for which can be seen be seen below.
Item Value Threshold Converged? Maximum Force 0.000008 0.000450 YES RMS Force 0.000005 0.000300 YES Maximum Displacement 0.000036 0.001800 YES RMS Displacement 0.000024 0.001200 YES
Frequency Analysis
The log file can be found: DOI:10042/202318 and for the frequency analysis: 10042/202323 DOI:10042/202323
Low frequencies --- -2.3055 -0.0029 -0.0018 0.0774 0.7534 0.7534 Low frequencies --- 155.9402 155.9405 267.6894
Jmol Dynamic Image
BBr3 |
Ammonia-Borane Association Energies
E(NH3): -56.55776 a.u Ng611 (talk) 22:16, 15 May 2018 (BST) To 6 dp, this was -56.557768, so you should have rounded to -56.55777, rather -56.55776
E(BH3): -26.61532 a.u
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)]= -0.05580 a.u= -139.5 KJ/mol
The association energy points to a weak bond. This can be justified by comparing to the association energy of the carbon carbon single bond, which has an association energy of 618+-15.4 KJ/mol.1
Ref: 1. https://notendur.hi.is/agust/rannsoknir/papers/2010-91-CRC-BDEs-Tables.pdf
Ng611 (talk) 22:15, 15 May 2018 (BST) Remember to cite your bond values from a textbook, databook, or paper, rather than a website, and remember that you should round your answers to the nearest Kj/mol
Part 2: Mini project- Aromaticity
Optimisation
Benzene and Borazine were optimised using the B3LYP and the 6-31G with results shown below
Benzene Item Value Threshold Converged? Maximum Force 0.000198 0.000450 YES RMS Force 0.000082 0.000300 YES Maximum Displacement 0.000849 0.001800 YES RMS Displacement 0.000305 0.001200 YES
Borazine Item Value Threshold Converged? Maximum Force 0.000085 0.000450 YES RMS Force 0.000033 0.000300 YES Maximum Displacement 0.000249 0.001800 YES RMS Displacement 0.000077 0.001200 YES
Frequency Analysis
Benzene
Low frequencies --- -0.0010 -0.0008 -0.0005 55.9811 56.8433 59.3704 Low frequencies --- 421.8710 422.0833 626.3430
Borazine
Low frequencies --- -4.4947 0.0004 0.0008 0.0008 7.0837 9.2341 Low frequencies --- 289.5756 289.7008 404.3392
Jmol Images
Benzene |
Borazine |
Charge Distribution Comparison
A charge distribution comparison between benzene and borazine is presented below:
| Benzene | Borazine |
|---|---|
 |

|
| 0.239 (Hydrogen) | -0.747 (Hydrogen bound to Boron), 0.432 (Hydrogen bound to Nitrogen) |
| -0.239 (Carbon) | 0.747 (Boron), -1.102 (Nitrogen) |
The different charge distributions observed can be explained based on the different electronegativities of the atoms involved. In the case of benzene, carbon is more electronegative than hydrogen leading to a negative charge being developed on the carbon and a positive one on the hydrogens. In the case of borazine, however, the fact that nitrogen is more electronegative than hydrogen bur boron is less leads to a negative charge on hydrogens bound to boron atoms and a positive one on those bound to nitrogen. The smaller electronegativity difference between hydrogen and boron compared to hydrogen and nitrogen leads to a more even distrubution between the former compared to the latter. For this comparison the NBO (Natural Bond Orbital) charge distributions were used, which represent the relative localisation of electron density on each atom.
MO Comparison
Aromaticity
Aromatic compounds are more stable compared to their unsaturated analogues or other configuration of the same set of atoms. This can be attributed to their cyclic conjugated system of overlapping p orbitals that allows electron delocalisation of electrons over the cyclic system. This electron delocalisation of electrons gives rise to a ring current in the presence of an external magnetic field. The display bond lengths of values between those for single and double bonds. Despite earlier theories that included planarity as a prerequisite for aromaticity, it has now been demonstrated that non-planar molecules can be aromatic.
Both benzene and borazine display this conventional concept of aromaticity. Benzene has six carbons, each contributing a single electron to the delocalised pi system. Borazine has three nitrogens, each contributing an electron pair and three borons that each posses an empty orbital. As a result, they both obey the conventional Huckel rule for aromaticity that requires 4n+1 electrons in a planar, conjugated cyclic system. Benzene, however, is significantly more aromatic, a fact that is reflected on its greater stabilisation.
However, the concept of aromaticity is not without controversy. A number of criteria have been developed to try and predict whether a molecule is aromatic. It has been demonstrated that even the same criteria do not always lead to the same outcome. Moreover, the connection of the concept with the structure and properties of benzene is problematic. Recent advances in chemistry have demonstrated that molecules with significant structural differences to benzene display stabilisation. This has given rise to concepts such as quasi- and pseudo- aromaticity that describe the situations where compounds satisfy some but all criteria and compounds that resemble aromatic structures but are not so2. This leads to the conclusion that there is a quantum mechanic basis to aromaticity.
As seen above, overlapping of p orbitals gives rise to aromaticity. However, this is not sufficient to describe all the situations in which aromaticity can arise.
Ng611 (talk) 22:20, 15 May 2018 (BST) Good discussion!
Ng611 (talk) 22:20, 15 May 2018 (BST) Overall a very solid report. You needed to include MO snapshots of all 7 orbitals, as opposed to simply the HOMO/LUMO. A better comparison for assiciation energies would also be good -- the bond enthalpy you cited is actually for a C=C bond, not a single bond. Your aromaticity section was very good -- describing the symmetries and constituent AOs that comprise the benzene and borazine MOs would have improved it further still!
References
1. https://notendur.hi.is/agust/rannsoknir/papers/2010-91-CRC-BDEs-Tables.pdf
2.https://onlinelibrary.wiley.com/doi/epdf/10.1002/chem.200700250