Nk1016InorgCCL
Year 2 Inorganic Computational Chemistry Lab
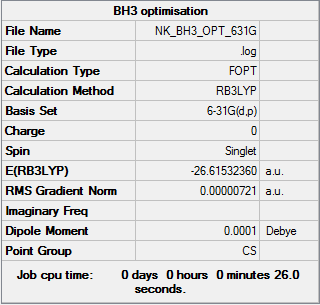
BH3
B3LYP/6-31G level
Item Value Threshold Converged? Maximum Force 0.000012 0.000450 YES RMS Force 0.000008 0.000300 YES Maximum Displacement 0.000064 0.001800 YES RMS Displacement 0.000039 0.001200 YES
Frequency analysis log file Media:NK1016_BH3_FREQ.LOG
Low frequencies --- -10.0599 -3.0201 -0.0054 0.4917 2.0912 3.6735 Low frequencies --- 1162.9537 1213.1540 1213.1567
BH |
| Vibration | Intensity | Symmetry | IR Active? | Type |
|---|---|---|---|---|
| 1163 | 93 | A2 | Yes | Bend |
| 1213 | 14 | E' | Yes | Bend |
| 1213 | 14 | E' | Yes | Bend |
| 2582 | 0 | A' | No | Stretch |
| 2716 | 126 | E' | Yes | Stretch |
| 2716 | 126 | E' | Yes | Stretch |
There are less than 6 peaks as one vibration is not IR active, and there are 2 pairs of degenerate vibrations so only 3 peaks are visible.
Smf115 (talk) 13:23, 25 May 2018 (BST)Correct symmetry assignments and mention of both the IR inactive and degenerate modes. To improve, a bit more detail about the modes themselves rather than just stretch or bend could be included and a more developed comment.
MO Diagram:
The real and LCAO MOs are very similar in what they represent.
i.e. They both show the same nodes and give similar electron densities.
This shows that qualitative MO theory is very useful when it comes to predicting "real" MOs.
Ammonia-Borane:
E(NH3) = -56.55777 a.u.
E(BH3) = -26.61532 a.u.
E(NH3BH3) = -83.22469 a.u.
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)]
ΔE = -0.05160 a.u. (-140 KJ/mol)
This is a sensible value for the bond energy as you expect it to be around a couple hundred kilo-joules per mole.
I would say that the B-N dative bond is relatively weak as an N-N single bond has an energy of ~167 KJ/mol while an N-N triple bond (one of the strongest bond around) has an energy of ~942 KJ/mol.
Smf115 (talk) 13:23, 25 May 2018 (BST)Correct calculation and good comparisons used. To improve literatue values should be referenced and structure information should have been included for both NH3 and NH3BH3.
BBr3
B3LYP/6-31G(d,p)LANL2DZ level
Item Value Threshold Converged? Maximum Force 0.000013 0.000450 YES RMS Force 0.000006 0.000300 YES Maximum Displacement 0.000051 0.001800 YES RMS Displacement 0.000035 0.001200 YES
Frequency analysis log file DOI:10042/202419
Low frequencies --- -0.0002 -0.0002 -0.0001 49.7266 49.8710 50.0368 Low frequencies --- 144.7443 144.7665 215.6321
BBr |
Lewis acids and bases
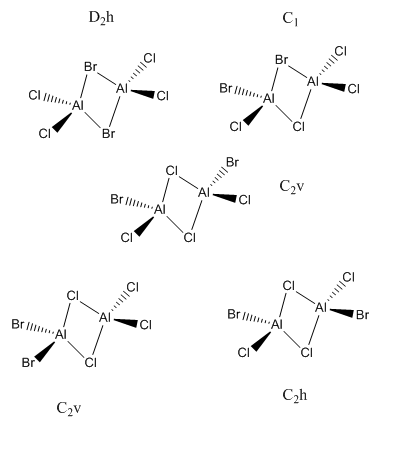
There are 5 possible isomers of Al2Cl4Br2 and these are shown below:
Smf115 (talk) 12:36, 27 May 2018 (BST)Correct symmetry assignments and isomers.
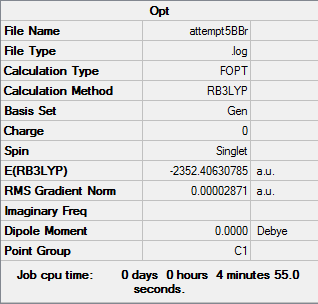
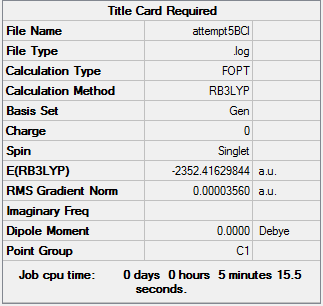
With the isomer with 2 bridging Br ions as isomer (a) and the isomer with 2 bridging Cl ions and trans terminal Br as isomer (b), here are the optimized data for isomer (a) and (b).
B3LYP/6-31G(d,p)LANL2DZ level
Isomer (a):
Item Value Threshold Converged? Maximum Force 0.000068 0.000450 YES RMS Force 0.000028 0.000300 YES Maximum Displacement 0.001359 0.001800 YES RMS Displacement 0.000586 0.001200 YES
Isomer (b):
Item Value Threshold Converged? Maximum Force 0.000072 0.000450 YES RMS Force 0.000028 0.000300 YES Maximum Displacement 0.001169 0.001800 YES RMS Displacement 0.000404 0.001200 YES
Using the energies calculated, isomer b can be found to be 0.00999 a.u. (26 KJ/mol) more stable than isomer a.
This shows that a smaller bridging ion like Cl gives a more stable compound than a larger bridging ion like Br.
Dissociation Energy: 0.03606 a.u. (90 KJ/mol)
Smf115 (talk) 12:36, 27 May 2018 (BST)The correct calculations have obviously been performed and it appears that the correct pseudopotential has been used. However, full structural infomration and links to the frquency log files were expected for both isomers and for the monomer. The calculations themselves should be included and a reason for why isomer a is more stable would have developed your answer further.
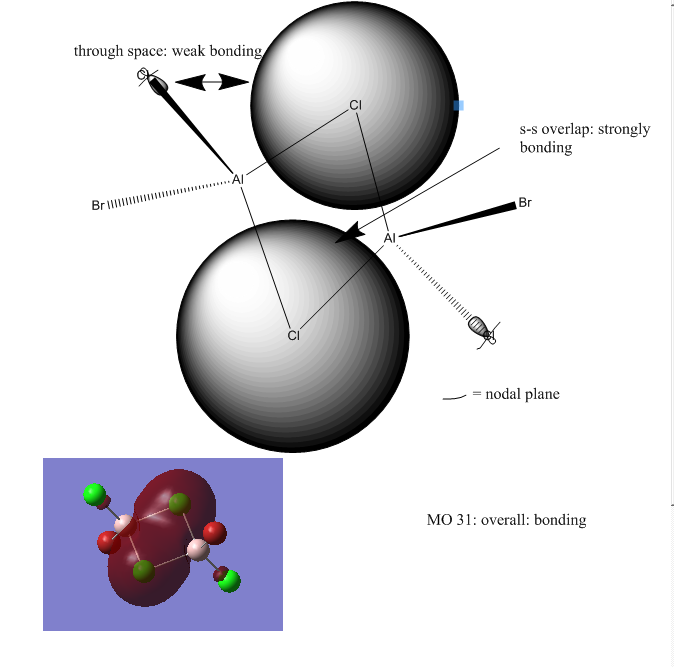
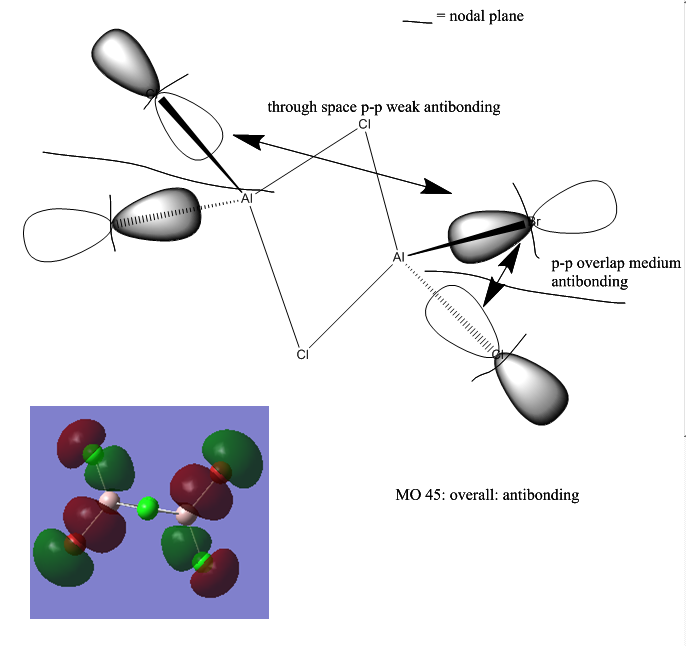
Further analysis was performed on isomer b (the lowest energy isomer) and its MOs were visualised:
Smf115 (talk) 12:36, 27 May 2018 (BST)Overall a decent attempt, however, a proportion of required information is missing and answers to questions require more detail an thought throughout.