Nitroglycerin
Nitroglycerin
| Nitroglycerin | |
|---|---|
== | |
| General | |
| Systematic name | propane-1,2,3-triyl trinitrate |
| Other names | Nitroglycerine (UK English spelling) |
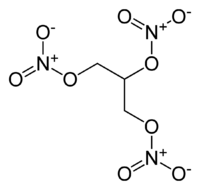
| Molecular formula | C3H5(NO3)3 |
| SMILES | {{{SMILES}}} |
| Molar mass | 227.09 g/mole |
| Appearance | oily, colourless liquid |
| CAS number | {{{CASNo}}} |
| Properties | |
| Density & phase | {{{Density}}} g/cm³ |
| Solubility in water | {{{Sol_Water}}} g/100 ml (25°C) |
| Melting point | 13.2 degrees Celcius (286.2 K) |
| Boiling point | {{{Bp}}} K |
| Acidity (pKa) | {{{pKa}}} |
| Basicity (pKb) | {{{pKb}}} |
| Chiral rotation [α]D | {{{Rotation}}}° |
| Viscosity | {{{Viscosity}}} cP at 25°C |
| Structure | |
| Molecular shape | acyclic |
| Coordination geometry |
{{{Coordination}}} |
| Crystal structure | {{{Crystal_Structure}}} |
| Dipole moment | {{{DM}}} D |
| Hazards | |
| MSDS | External MSDS |
| Main hazards | Extremely unstable, very explosive |
| NFPA 704 | {{{NFPA}}} |
| Flash point | {{{Fp}}}°C |
| R/S statement | R: {{{R-S}}} S: ? |
| RTECS number | {{{RTECS}}} |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Other anions | {{{Other_anion}}} |
| Other cations | {{{Ohter_cation}}} |
| Related compounds | {{{Relative_Compounds}}} |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
| Structure | |
|---|---|
| Molecular shape | {{{MolShape}}} |
| Coordination geometry |
{{{Coordination}}} |
| Crystal structure | {{{CrystalStruct}}} |
| Dipole moment | {{{Dipole}}} D |
Nitroglycerin was created by in 1847 by Ascanio Sobrero at the University of Turin. However, Alfred Nobel found the best method of producing it in the 1860’s. Due to its unstable nature, nitroglycerin was banned and, in turn, led to the production of dynamite by mixing together inert absorbing agents with the nitroglycerin. Nobel used kieselguhr, which is also known as diatomaceous earth, and was one of the first producers of dynamite. Due to this discovery, and its inevitable use in World War One, Nobel created the Nobel prizes to refrain people from remembering him as the scientist who caused mass distruction.
It is a colourless and oily compound that has properties that make it a very powerful explosive reagent. The atoms that are contained in nitroglycerin are nitrogen, oxygen and carbon, which produce stable molecules with strong bonds when the compound explodes (for example nitrogen gas, oxygen gas, steam and carbon monoxide gas) and this stability is the driving force of the reaction. The reaction liberates a large amount of heat because the strong bonds in the product gas molecules replace the fewer, weaker bonds in nitroglycerin.
Why is Nitroglycerin explosive?
The explosive force of nitroglycerin is due to pressure build up from its decomposition into the hot gases. The reaction of detonation is as follows:
4C3H5N3O9(s) 6N2(g) + 12CO(g) + 10H2O(g) + 7O2(g)
This shows that 35 moles of gases is produced from 4 moles of nitroglycerin. Nitroglycerin is produced commercially by mixing sulphuric acid and nitric acid in a 1:1 ratio. Hydrocarbons usually burn instead of exploding because oxygen must come into contact with the fuel in the combustion reaction. Nitroglycerin creates its own oxidant which in turn means that oxygen doesn't have to come into contact with the fuel to keep the reaction going.
References
http://www.ch.ic.ac.uk/rzepa/mim/environmental/html/nitroglyc_text.htm
The Merck Index, 8th ed., Merck & Co., Rahway NJ, 1968.
