Nattan Yeshitila 1343100
NH3 Optimisation
Summary Results
- Molecule: NH3
- Calculation Method: RB3LYP
- Basis Set: 6-31G(d.p)
- E(RB3LYP): -56.55776873 a.u.
- RMS Gradient: 0.00000485 a.u.
- Point Group: C3V
- Bond Length: 1.01798 A
- Bond Angle: 105.7410
Item Table
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Predicted change in Energy=-5.986277D-10
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.018 -DE/DX = 0.0 !
! R2 R(1,3) 1.018 -DE/DX = 0.0 !
! R3 R(1,4) 1.018 -DE/DX = 0.0 !
! A1 A(2,1,3) 105.7412 -DE/DX = 0.0 !
! A2 A(2,1,4) 105.7412 -DE/DX = 0.0 !
! A3 A(3,1,4) 105.7412 -DE/DX = 0.0 !
! D1 D(2,1,4,3) -111.8571 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
Dynamic Image
NH3 |
This image can be found (File:NY517 NH3 OPTIMISATION 3.LOG)
Vibrational and Frequency Analysis
Questions
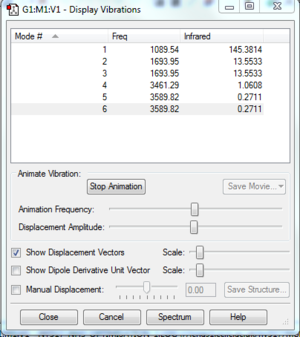
Since NH3 is a non-linear molecule, the number of nodes should follow the 3N-6 rule where N represents the number of atoms. As a result, I would expect the molecule to have 6 nodes which is what was produced.
Modes 2&3 and 5&6 are degenerate because they have the same frequency and hence the same energy. The frequency of their vibrations are the same because the vibrations have the same motion but occur on different bonds in the opposite directions.
Modes 1-3 are bending vibrations and modes 4-6 are stretching vibrations. This could have been predicted since stretching vibrations are generally a higher energy than bending vibrations.
Mode 4 is highly symmetric because the bonds stretch in and out simultaneously and therefore maintain their symmetry through the the central atom. This is called a symmetric stretch.
Mode 1 is the umbrella mode due to the bending vibration of the bonds up and down simultaneously.
I would expect to see four bands in an experimental spectrum where the peaks at 3461.30 and 3589.86 would be so small that they are unlikely to be spotted clearly in the spectrum.
Charges
Nitrogen atom: -1.125 Hydrogen atoms: +0.375
I would have expected nitrogen to be negatively charged and hydrogen to be positively charged because nitrogen is more electronegative than hydrogen. Also, the total charge of the molecule is zero which is expected since it is a neutral molecule.
Haber-Bosch Process (N2 & H2)
N2
Summary Results
- Molecule: N2
- Calculation Method: RB3LYP
- Basis Set: 6-31G(d.p)
- E(RB3LYP): -109.52412868 a.u.
- RMS Gradient: 0.00000060 a.u.
- Point Group: D*H
- Bond Length: 1.10550 A
- Bond Angle: 1800
Item Table
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
Predicted change in Energy=-3.401007D-13
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.1055 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
Dynamic Image
N2 |
This image can be found (File:NY517 N2 OPTIMISATION.LOG)
Vibrational and Frequency Analysis
H2
Summary Results
- Molecule: H2
- Calculation Method: RB3LYP
- Basis Set: 6-31G(d.p)
- E(RB3LYP): -1.17853936 a.u.
- RMS Gradient: 0.00000017 a.u.
- Point Group: D*H
- Bond Length: 0.74279 A
- Bond Angle: 1800
Item Table
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
Predicted change in Energy=-1.164080D-13
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 0.7428 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
Dynamic Image
H2 |
This image can be found (File:NY517 H2 OPTIMISATION.LOG)
Vibrational and Frequency Analysis
Energy Values (au)
- E(NH3)= -56.55776873
- 2*E(NH3)= -113.11553746
- E(N2)= -109.52412868
- E(H2)= -1.17853936
- 3*E(H2)= -3.53561808
- ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.05579070= -146.48 kJ mol-1
The formula for ΔE in this reaction is applicable because 2 moles of NH3 are formed when 1 mole of N2 reacts with 3 moles of H2. Hence why the energy in bold is the energy for converting hydrogen and nitrogen gas to ammonia. Ammonia is more stable than the gaseous reactants because it has a lower energy structure.
O2 Optimisation
Summary Results
- Molecule: O2
- Calculation Method: RB3LYP
- Basis Set: 6-31G(d.p)
- E(RB3LYP): -150.25742434 a.u.
- RMS Gradient: 0.00007502 a.u.
- Point Group: D*H
- Bond Length: 1.01798 A
- Bond Angle: 105.7450
Item Table
Item Value Threshold Converged?
Maximum Force 0.000130 0.000450 YES
RMS Force 0.000130 0.000300 YES
Maximum Displacement 0.000080 0.001800 YES
RMS Displacement 0.000113 0.001200 YES
Predicted change in Energy=-1.033738D-08
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.216 -DE/DX = -0.0001 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
Dynamic Image
O2 |
This image can be found here (File:NY517 O2 OPTIMISATION.LOG)
Vibrational and Frequency Analysis
Molecular Orbitals
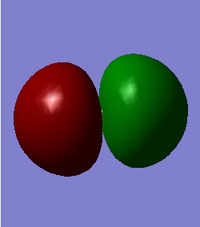
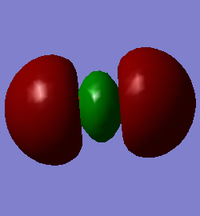
This is a σ*s orbital formed from the combination of 2s orbitals from both atoms out of phase. It has a node in the middle resulting in a positive and negative phase. It is an anitbonding orbital deep in energy (at -0.798 au) and is fully occupied. This orbital does not really affect reactivity due to its very low energy.
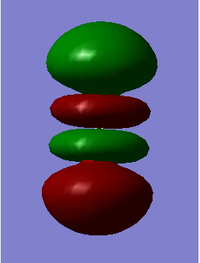
This is a σpz orbital formed from the overlap of 2pz orbitals from both oxygen atoms. It has two nodes caused by the overlapping of the p-orbitals and the fact that p-orbitals already have a node. It is a bonding orbital that is fully occupied and has an energy of -0.532 au. sp mixing occurs between this orbital and the πp orbitals due to them being similar in energy and the orientations of the orbitals being slightly aligned for overlapping.
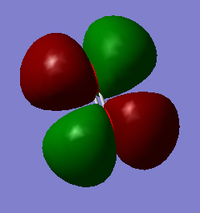
Depending on the axis, this could be a πpx or πpy orbital. They have identical shapes but just in a different orientation. It has one node formed from the orthogonal overlapping of px or py degenerate orbitals hence why it has a different shape to the σpz orbital. Despite being formed from degenerate orbitals, their energies are very slightly different most likely due to the different levels of sp mixing. This is a bonding orbital that is fully occupied in the x and y orientations.
Once again, depending on the axis, this could be a πpx* or πpy* orbital. This has four nodes caused by the of degenerate p-orbitals. This was once again an orthogonal overlap but is now an antibonding orbital hence why the shape is different to that of px and py. It results in more nodes and more more changes in phase. These represent the HOMO orbital and since they are only half full, any addition of electrons will be added to these orbitals and so they are very reactive. If an electron was to be excited, it would be excited from these orbitals to the LUMO.
This is a σpz* orbital formed from the out of phase overlap from 2pz orbitals. There are four different phases in this despite even though only two would be predicted. This is due to the sp mixing with causing these extra nodes. This is an antibonding orbital that is unfilled and is hence the LUMO. The HOMO-LUMO is fairly small therefore an excitation can be likely with relatively low energy.