Namespace:jr4072PROJECT

Mini-Project: 3rd. yr. Computational Chemistry Lab - Inorganic (Module 2)
A mini-project in inorganic computational chemistry has been conducted based around thiophene polycyclic based semi-conductors, illustrating the use of MOs, and spectral predictions in determining the structure, bonding and properties of molecules.
Modelling Techniques & Calculations
Computational models are built from ChemDraw / Chem3D and GaussView. Optimisations are run by MM2 (molecular mechanics), then PM6 (semi-empirical molecular orbital theory) in Chem3D, followed by Gaussian quantum calculations - solving the Schroedinger equation - giving a more accurate result (for a defined basis set).
Thiophene Polyaromatic Based Semi-Conductors

This project considers the properties of semi-conductors, taking a synthesised semi-conductor from the literature, and assessing it against its isomer and a derivative, in an attempt to identify a good semi-conductor.
The ideal properties of a semi-conductor are:
- Crystallinity - for practical handling at room temperature
- Planarity - for packing efficiency and delocalisation
- Delocalisation / conjugation - for conductivity
- High molecular weight - higher melting point and more stable
- Good band gap - achieved by use of Si groups
These may be investigated by:
- Visual inspection of structure / planarity and measurement of dihedral angles
- Vibrational analysis (to confirm optimised structures) probing conjucation (through bond strength)
- MO analysis visually assessing delocalisation and semi-conductor nature
- UV analysis: to make some approximations about the band gap
Background

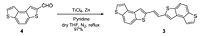
The (potential) semi-conductors A/B are for use as organic thin-film transistors (OTFTs), cheaper alternatives to amphorous Si based TFTs with more potential applications: molecules C/D are a hybrid. OFTs have use in several fields, including flexible displays, low-cost electronic paper, smart textiles, and sensor elements in the automotive industry.
The semi-conductor materials is layered with a dielectric onto a substrate (e.g. SiO2/Si), being connected by electrodes (termed source S and drain D), typically in a 'top-contact' configuration, where the electrode (e.g. Au) is in contact with the top of the semi-conductor material. This said, a 'bottom-contact' device is easier to manufacture and the ultimate goal. The device is then activated when a voltage is applied between the source and a gate (G) electrode. A schematic of the construction is shown below, with additional examples of semi-conductor materials.
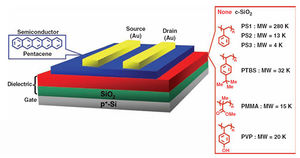
The semi-conductor is optimised when the field-effect mobility and on/off current (D-S) ratio are as high as possible. Molecule A is reported in the literature[4], with mobilities of 4x10-4-2x10-2cm2/Vs (HMDS substrate) and current ratio of 106-107, at an optimum temperature of 55degC, performance at different temperatures being attributed to changes in film crystallinity and morphology - higher crystallinity gives better performance.
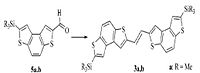
Molecules A and B are readily synthesised in a McMurry procedure (shown right)[5] with C and D being synthesised in an analogous scheme with a modified precursor.[6]
Optimisation & Structural Analysis
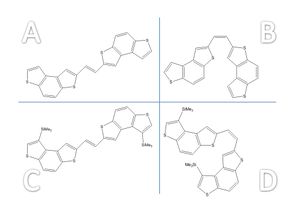
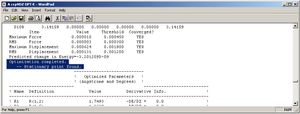
A calculation was run for each molecule to optimise its geometry: the structures of the four molecules are shown right, with links to 3D JMol visuals (optimised). These were run on the DFT RB3LYP method with a ccpVDZ basis set, pre-determined to be the most accurate calculation.
The basis set was chosen by optimising each molecule across a range of basis sets, as shown in the table below, and comparing the energy and dipole moments found. Before Gaussian optimisation each molecule was optimised by MM2 and then PM6 methods.

The simplest basis set is the 3-21G, replacing with the LANL2MB set (to model core potentials) seems poor here, increasing dipole moments; furthermore 6-31G (using more functions) gives similar dipole moments and lower energies - more stable structures, the use of core potentials at a comparative level (LANL2DZ) is now effective, reducing the dipole moments for all molecules. Finally the more accurate ccpVDZ set gives lower energies and dipole moments, producing the most stable structures. Addition of keywords to refine the calculation takes this a step further. The graph of overall dipole moment for each molecule varying with basis set shows that ccpVDZ (with keywords) produces the most optimal results.
The optimisation results (energies and structural comparisons) are tabulated below. The crucial difference between the molecules is the deviation from planarity for cis isomers, with bulky steric groups enhancing this as illustrated by the variation of central dihedral angle (graphed below).

Discussion
Notably the C=C bond length is similar in all cases, and there is little energy difference between the cis and trans conformers. The essential difference is the presence of a real dipole moment for the cis conformer (illustrated above), as this bends away from planarity, bringing the electronegative sulphur atoms on to one side of the molecule. The deviation from planarity critically means that the molecule would not pack well, making a poor semi-conductor. A similar argument could be applied to molecule D compared to A, though it is assumed that the SiMe3 groups, though bulky, confer stability to the molecule, making it a higher molecular weight and better semi-conductor. Additionally, as aforementioned, the Si groups help to produce the desired band gap.
In the McMurry synthesis of A (shown above, also of B by isomerism) the literature reports a trans/cis (95/5) mixture, the trans structure being the more favourable.[7]
Vibrational Analysis
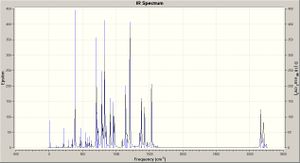
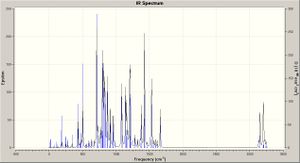

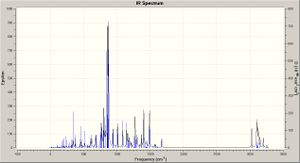
Taking the optimised structures a vibrational analysis was run for each molecule (DFT, RB3LYP, ccpVDZ; int=ultrafine scf=conver=9): the spectra are shown right. The spectum for molecule A is analysed below.

Critically the low vibrations are all close to zero (-2.4245 -1.3683 -0.0008 0.0021 0.0023 0.6040), indicating a good degree of accuracy, and there are no negative vibrations, thus a minimum energy structure has been achieved. Owing to the large number of atoms there are many possible vibrations, the most intense being illusrated. The spectum for molecule B is analysed below.
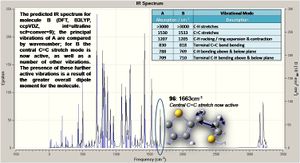
Many of the vibrations for A and B are highly similar, the cis conformer produced dipole does however lead to activation of the C=C stretch. Furthermore, as shown in the comparison table, the vibrations of B are often at slightly lower frequencies, corresponding to lower energies and weaker bonds: from this one expects the delocalisation across the molecule to be reduced by the divergence from planarity (as orbitals can no longer align so well).
The literature quotes an IR absorption at 1645cm-1 for the trans/cis (95/5) mixture, though it is hard to assess which absorption this would correspond to, as there are no intense peaks in the predicted spectra for this region, and the only peak (weak) is for the cis C=C stretch, though the literature reports a majority of trans product so one would expect this peak to be further reduced in intensity.[8]
The spectum for molecule C is analysed below.
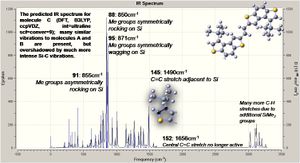
The introducion of the Si groups produces much more intense vibrations, with those modes of molecule A now being overshadowed. The spectum for molecule D is analysed below.
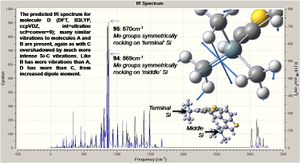
Molecule D has similar vibrations to B, but like C the Si based modes are now the most intense, overshadowing all others; the central C=C stretch is active again.
Discussion
Vibrational analysis confirms the structures to be minimal energy optimisations (no negative frequencies). There are a great number of vibrations present, due to the many atoms in each of the molecules. The intensity of the central C=C stretch illustrates the necessity of a dipole moment for an absorption to be IR active (occuring only for the cis conformer). Si based absorptions are also far more intense than C based, associable with the greater molecule weight of Si requiring more energy to vibrate. The cis conformers are shown to be less stable through lower absorption frequencies, presumed to arise from less delocalisation due to the loss of planarity - this is reflected in the differing extent of orbital overlap between the isomers (see NBO analysis below).
MO Analysis
Using the optimised geometry structures (obtained above) NBO analysis calculations were run for each molecule (DFT, RB3LYP, ccpVDZ; pop=full,nbo). The results are summarised below, giving the qualitative MOs of each molecule.

The MOs show a large degree of delocalisation across the molecule, with a suprising number of nodes for the HOMO (conferring a high energy: the more nodes the higher the energy). In addition, the MOs all show high degrees of pi bonding, with electron clouds stretching across several atomic centres, above and below the plane (with phase inversion). Furthermore the MOs are all symmetrical with a point of inversion at the centre of the central C=C bond - this reflects the symmetry of the molecular structure, and the absence of the C=C stretch on the predicted IR spectrum.
On going from the HOMO to the LUMO regions of bonding become regions of anti-bonding: for example about the central C=C bond.
Notably the molecule has four MOs (LUMO, +1, +2, +3) that are all negative in energy, and thus can accept electrons without destabilising the molecule. This makes the molecule a good p-type (electron defficient) semi-conductor.

The MOs of B are somewhats similar to A, being typically the same on one side of the molecule, there is now however a notable break in the symmetry of the MO about the central C=C bond. MOs are similarly delocalised with high levels of pi bonding, but the electron clouds span less atomic centres (well shown in HOMO-2, centering more electron density on one side of the molecule, in agreement with the calculated overall dipole moment).
As with A the first four UMOs are also negative; the HOMO-LUMO energy gap is now bigger (discussed below).

The MOs of C are very similar to A, showing all of the same trends, with a similar HOMO-LUMO gap.

The comparison of D to C is as of B to A: the symmetry in the MOs is lost, due to cis isomerism; the HOMO-LUMO gap is increased further by the bulky steric Si groups, while the difference in energy between each OMO is reduced in comparison to A.
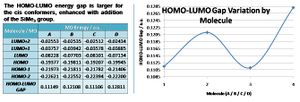
The HOMO-LUMO gap variation for the molecules: this is tabulated below and graphed. We see the gap is larger for the cis conformers, this being enhanced by the addition of the SiMe3 groups.
Discussion
The qualitative visual MOs of all four molecules have many nodes being at high energy levels, but also largely delocalised across the molecules. The MOs of the trans species display symmetry which is broken in the cis isomers, giving slightly less stability.
Critically all four molecules have four UMOs (LUMO, +1, +2, +3) with negative energies, which stabilise the molecule when occupied by electrons, making for plausible p-type (electron defficient) semi-conductors. Equally the cis conformer has a greater HOMO-LUMO gap, the addition of SiMe3 groups enhancing this.
The calculated HOMO-LUMO gap (band gap) for molecule A is 3.03eV (0.11189a.u.), where the literature[9] reports an estimated value of 2.7eV, taking 10% of the maximum for low energy band edges. Indeed the exact HOMO-LUMO gap is not necessarily the energy of excitation due to solvent, solid state, and stokes-shift effects for example. The process of excitation from the HOMO to the LUMO in a semi-conductor is illustrated below.
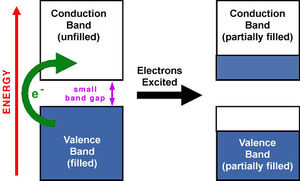
NBO Analysis
The calculations to determine the MOs also allow for a natural bond analysis, identifying the AOs involved in bonding, and of more concern the overlap between orbitals, that helps to stabilise the molecule and extend delocalisation.
The orbital overlap of concern (>20kcal/mol) for molecule A is shown below:
Second Order Perturbation Theory Analysis of Fock Matrix in NBO Basis
Threshold for printing: 0.50 kcal/mol
E(2) E(j)-E(i) F(i,j)
Donor NBO (i) Acceptor NBO (j) kcal/mol a.u. a.u.
===================================================================================================
within unit 1 1. BD ( 1) C 1 - S 2 /124. RY*( 2) C 3 0.68 1.56 0.029 98. LP ( 2) S 2 /389. BD*( 2) C 1 - C 5 21.33 0.27 0.069 100. LP ( 2) S 10 /409. BD*( 2) C 11 - C 12 20.75 0.27 0.067 102. LP ( 2) S 16 /419. BD*( 2) C 15 - C 19 20.75 0.27 0.067 104. LP ( 2) S 26 /437. BD*( 2) C 24 - C 25 21.33 0.27 0.069 404. BD*( 2) C 8 - C 9 /399. BD*( 2) C 6 - C 7 252.74 0.01 0.079 409. BD*( 2) C 11 - C 12 /413. BD*( 2) C 13 - C 14 124.56 0.01 0.074 419. BD*( 2) C 15 - C 19 /413. BD*( 2) C 13 - C 14 124.56 0.01 0.074 422. BD*( 2) C 17 - C 18 /428. BD*( 2) C 20 - C 21 252.74 0.01 0.079
The interactions of the S lone pairs with the C=C anti-bonding orbitals (98-104) are shown below.
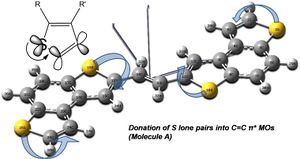
Molecule B shows similar overlaps:
Second Order Perturbation Theory Analysis of Fock Matrix in NBO Basis
Threshold for printing: 0.50 kcal/mol
E(2) E(j)-E(i) F(i,j)
Donor NBO (i) Acceptor NBO (j) kcal/mol a.u. a.u.
===================================================================================================
within unit 1 1. BD ( 1) C 1 - S 2 /124. RY*( 2) C 3 0.67 1.56 0.029 98. LP ( 2) S 2 /389. BD*( 2) C 1 - C 5 21.26 0.27 0.069 100. LP ( 2) S 10 /409. BD*( 2) C 11 - C 12 20.29 0.27 0.066 102. LP ( 2) S 19 /418. BD*( 2) C 15 - C 16 20.88 0.27 0.067 104. LP ( 2) S 26 /437. BD*( 2) C 24 - C 25 21.40 0.27 0.069 404. BD*( 2) C 8 - C 9 /399. BD*( 2) C 6 - C 7 295.20 0.01 0.079 409. BD*( 2) C 11 - C 12 /413. BD*( 2) C 13 - C 14 76.54 0.01 0.060 418. BD*( 2) C 15 - C 16 /413. BD*( 2) C 13 - C 14 63.38 0.02 0.068 423. BD*( 2) C 17 - C 18 /433. BD*( 2) C 22 - C 23 297.52 0.01 0.079 428. BD*( 2) C 20 - C 21 /433. BD*( 2) C 22 - C 23 297.16 0.01 0.079
The interactions of the various C=C anti-bonding orbitals are illustrated below.

The results for molecules C and D are near identical, with the same overlaps (just at different energies, but all still above 20kcal/mol).
Discussion
All molecules show the same pi based orbital overlap interactions, mainly from (a) S lone pair donation into adjacent C=C double bonds, (b) electron rich C=C double bonds (between thiophene/benzene rings) into electron poor double bonds on benzene (this forms a dipole as electron density builds up on one side of the molecule in the cis conformation).
Further donation occurs (c) from thiophene C=C double bonds into the central C=C double bond: the extent (energy) of this is greater for molecule A than B, due to better aligned orbitals (planar structure); as with the IR spectra and MOs this confirms a greater extent of delocalisation in the trans conformer.
Overall mixing of orbitals helps to stabilise molecule, lowering its overall energy, and increasing delocalisation.
Extensive delocalisation in the molecules as confirmed by the output .log file (example below for molecule A):
Structure accepted: RESONANCE keyword permits strongly delocalized structure
WARNING: 1 low occupancy (<1.9990e) core orbital found on C 3
UV Analysis

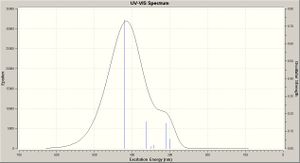
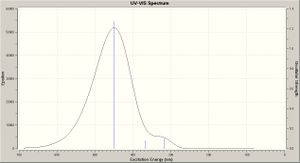
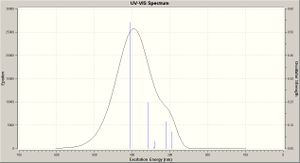
A time dependent calculation was run to predict the UV spectra for each molecule (TDSCF, DFT, RB3LYP, ccpVDZ; int=ultrafine scf=conver=9; N = 6 states), these however took >48 hours then being terminated by the server - UV-Vis spectra were still produced (shown right), however the accuracy of these is questionable since the calculation terminated before completion. These are compared below.

The results are in agreement with the MO analysis: the peaks for cis isomers B/D are at the lowest wavelengths (highest energy), corresponding to the biggest band gaps. Equally the peaks for A/C are very close (nm maxima), that for C being slightly lower, conferring a bigger band gap for the Si molecule, illustrating how functional group addition can tweak the band gap.
The literature[10] reports (in solution [CH2Cl2]) for molecule A, with absorption maxima at 370, 391 and 415nm. The latter (strong) associated with the E (trans) conformation. Here a prediction is of a peak maxima c.440nm, however bang gap results are much closer to literature values than with the MO analysis:
- A: peak maxima @ c.440nm - band gap 2.82eV
- B: peak maxima @ c.420nm - band gap 2.96eV
- C: peak maxima @ c.450nm - bang gap 2.76eV
- D: peak maxima @ c.395nm - band gap 3.94eV
The literature[11] reports a peak maxima at 424nm for the C/D mixture, interestingly (see above diagram) the absorption curves for the two separate isomers cross at c.420nm, so a mixture of the two should give a maxima at this point, which is thus in good agreement with the literature.
Discussion
The UV-Vis spectral prediction appears to have given a good approximation of the band gap for the molecules, with C (2.76eV) being closest to the literature values (c.2.7eV), suggesting this to be the best semi-conductor material by band gap.
Conclusions

The pairs of molecules A/B and C/D may both be formed by McMurry synthesis, with the trans product dominating, implying greater stability. However, geometric optimisation and analysis shows little energy difference between the isoemrs (to 2d.p. in a.u.); this said the cis conformer is bent away from planarity (trans is highly planar), generating a dipole moment which confers instability, illustrated by considering charge density. Calculations were performed on the ccpVDZ basis set, predetermined to be the most accurate (DFT, RB3LYP method; keywords: int=ultrafine scf=conver=9). Vibrational analysis confirms the structures to be minimal energy optimisations (no negative frequencies: frequency is second derivative of energy), also illustrating the lower energy of the cis conformer in some vibrations.
All four molecules are shown (MO/NBO analysis) to have strong orbital overlap and mixing, with highly conjugated systems and delocalisation; the HOMOs (inc. -1 and -2) are of high energy (many nodes and pi bonding), but the first four UMOs are of negative energy in all cases, making for p-type (electron defficient) semi-conductors. In the cis isomers the MOs lead to the formation of the dipole with an imbalance of electron density across the molecule, where as the trans isomer allows for better orbital alignment and overlap, giving more stable planar molecules. The HOMO-LUMO gaps are in the right region (~3eV) for semi-conductors for A/C but not (cis) B/D. This is further confirmed by UV-Vis analysis, which shows molecule C to have the most appropriate band gap (2.76eV).
The planar structure of A/C allows for better packing and crystallinity; the Si groups on C produce distinct differences in behaviour, as shown by the change in band gap, and push (steric bulk) the monomers apart, lowering stability. The literature further reports (as one would expects) a trans majority in the synthesis of A and B, isomerisation from A to be B requiring 'a highly diluted reaction mixture, a huge amount of solvent and long reaction times' due to the close packed plane structure. It is predicted that the introduction of substituents - such as those on C/D - reducing this packing, corroborating that these would pack less well. The literature quotes a melting point of 375degC for A/B mixture[12] and 332degC for the C/D mixture[13], confirming these suspicions. In addition, this has been probed by x-ray crystal structure determination for the similar molecule to C where SiMe3 groups are replaced with Si(iPr)3, shown right.
The choice of such elongated (6 rings) molecules is justified, the literature[14] confirming an increase in conjugation giving an increase in charge transport for organic semi-conductors.
In summary molecules A and C make for good semi-conductors, whereas B and C are not. A/C are planar allowing for better packing and crystallinity, with higher degrees of delocalisation/conjugation, giving high charge transfer, and band gaps in the appropriate region for semi-conductors. The addition of Si functional groups tweaks the bandgap and solubility/crystallinity. A UV-Vis analysis confirms the excitations occuring giving a better approximation of the band gap. The choice of either A or C for a device application would depend on the band gap desired, or conductance (improves with crystallinity), as these properties separate the two molecules apart.
Further Study
Wide-angle X-ray diffraction, atomic force microscopy and photoluminescence spectra are popular techniques to study such semi-conductor materials, allowing for surface investigations (plane packing etc) and band gap measurements.
Replacing the C=C central double bond with a benzene wrong connecting the adacent thiophene rings furnishes a helical structure, however such structures have been studied and found to lack semi-conducting ability. The cis isomers (B and D) are towards this helical structure, and thus it could be assumed that they they would lack conductivity. Highly organised films may be achieved by using benzene-sulphonate groups in the thiophene oligomer.[15] Indeed the crystallinity of molecules and packing is crucial in determining charge transfer, which can be aided by the choice of deposition technique, such as Langmuir Blodgett.
Calculation References
The calculation results have been deposited in d-space for reference.
The optimisation calculations may be found: A/B/C/D:
- with keywords: ccpVDZ basis set: DOI:10042/to-3104 DOI:10042/3120 DOI:10042/3133 DOI:10042/3121 ; and with the LANL2DZ basis set: DOI:10042/3106 DOI:10042/3105 DOI:10042/3118 DOI:10042/3119
- without keywords: 3-21G basis set: DOI:10042/3032 DOI:10042/3033 DOI:10042/3034 DOI:10042/3035 ; 6-31G basis set: DOI:10042/3052 DOI:10042/3051 DOI:10042/3050 DOI:10042/3049 ; the LANL2MB basis set: DOI:10042/3059 DOI:10042/3058 DOI:10042/3057 DOI:10042/3056 ; the LANL2DZ basis set: DOI:10042/3066 DOI:10042/3067 DOI:10042/3068 DOI:10042/3069 ; and finally the ccpVDZ basis set: DOI:10042/3074 DOI:10042/3075 DOI:10042/3076 DOI:10042/3077
The IR spectral predictions: (ccpVDZ; A/B/C/D): DOI:10042/to-3124 DOI:10042/to-3202 DOI:10042/to-3204 DOI:10042/to-3203
The NBO/MO analysis: (ccpVDZ; A/B/C/D): DOI:10042/3259 DOI:10042/3273 DOI:10042/3271 DOI:10042/3270
The UV-Vis spectral prediction (BH3): DOI:10042/3031 ; spectral (UV-Vis) predictions for A-D were not able to be published (to d-space), most likely to termination as opposed to completion.
Error Notes
Calculated energies are accurate to ~10kJ/mol - 0.003809a.u. - and are thus reported to 0.01a.u.; dipole moments are accurate to 0.01 Debye, and frequencies reported to 1cm-1 (typically 10% error), likewise intensities (infrared) are given as whole numbers; bond distances are accurate to 0.01a.u. and angles to 0.1deg.
Notably energies between molecules should only be considered on a relative (kJ/mol; not absolute!) level; absolute values are noted for reproducibility only (a.u.), and consistency is paramount for comparison (basis set, method, details).
Error analysis pre-determined: http://www.ch.ic.ac.uk/hunt/teaching/teaching_comp_lab_year3/8a_accuracy.html
References
- ↑ C. Kim et al., Organic Electronics, 10 (2009) 1511-1520
- ↑ S. Maiorana et al., Tetrahedron, 59 (2003) 6481-6488
- ↑ A. Bossi et al., Eur. J. Org. Chem., (2007) 4499-4509
- ↑ C. Kim et al., Organic Electronics, 10 (2009) 1511-1520
- ↑ S. Maiorana et al., Tetrahedron, 59 (2003) 6481-6488
- ↑ A. Bossi et al., Eur. J. Org. Chem., (2007) 4499-4509
- ↑ S. Maiorana et al., Tetrahedron, 59 (2003) 6481-6488
- ↑ S. Maiorana et al., Tetrahedron, 59 (2003) 6481-6488
- ↑ C. Kim et al., Organic Electronics, 10 (2009) 1511-1520
- ↑ C. Kim et al., Organic Electronics, 10 (2009) 1511-1520
- ↑ A. Bossi et al., Eur. J. Org. Chem., (2007) 4499-4509
- ↑ S. Maiorana et al., Tetrahedron, 59 (2003) 6481-6488
- ↑ A. Bossi et al., Eur. J. Org. Chem., (2007) 4499-4509
- ↑ F. Garnier et al., J. Am. Chem. Soc., 115 (1993) 19 8716
- ↑ F. Garnier et al., J. Am. Chem. Soc., 115 (1993) 19 8716
External links
- [Materials Research Science & Engineering Centres (USA): http://www.mrsec.org/]
- [Washington University (Department of Chemistry) - semi-conductors: http://www.chemistry.wustl.edu/~edudev/LabTutorials/PeriodicProperties/MetalBonding/images/semiconduction.jpg]
- [Chemistry SCAN Link: https://scanweb.cc.imperial.ac.uk/uportal2/]
- [Illustration of vibrational mode categories: http://en.wikipedia.org/wiki/Infrared_spectroscopy]
- [Online symmetry point group flow chart: http://capsicum.me.utexas.edu/ChE386K/Images/point_group_flowchart_shriver.jpg]
- [Main page: https://www.ch.ic.ac.uk/wiki/index.php/Namespace:jr4072]
