Naf:eezah
Module 1: Structure and Spectroscopy (Molecular Mechanics/Molecular orbital theory)
The Hydrogenation of the Cyclopentadiene Dimer
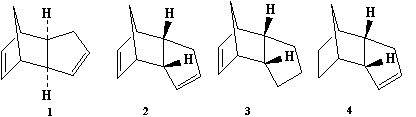
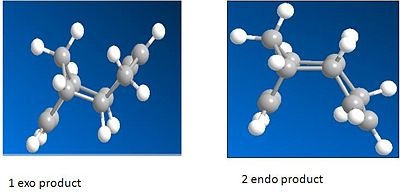
Cyclopentadiene dimerises to form the endo dimer not the exo dimer. Both dimmers were modelled using ChemBio 3D, the lowest energy geometry was found by using the MM2 force field option.

| Energy | exo1 | endo2 |
|---|---|---|
| Stretch | 1.27 | 1.25 |
| Bend | 20.6 | 20.8 |
| Torsion | 7.66 | 9.51 |
| 1,4 Van de Waals | 4.24 | 4.31 |
| Non 1,4 Van de Waals | -1.40 | -1.51 |
| Total Energy | 31.9 | 34.0 |
From these calculations it can be seen that the endo dimer has a higher total energy than the exo dimer even though it has a lower overall energy. This shows that the reaction is under kinetic control so the transition state for the endo dimer must be lower, the pathways are shown in the diagram above. The formation of the endo dimer is discussed in literature [1] and is due to the endo rule which is referred to as when there is "maximum acumulation of unsaturated centres."
Dehydrogenation of the dimer can form two products. These were modelled and the energies compared to see which one forms.

| Energy | product 3 | product 4 |
|---|---|---|
| Stretch | 1.27 | 1.10 |
| Bend | 19.8 | 14.6 |
| Torsion | 10.9 | 12.5 |
| 1,4 Van de Waals | 5.6 | 4.51 |
| Non 1,4 Van de Waals | -1.22 | -1.10 |
| Total Energy | 35.7 | 31.2 |
Product 3 is higher in energy than product 4. So product 4 is the most stable so it is formed. The energy calculated shows that the stretch energy is the highest in molecule 3 which shows the bond length is more deviated from the ideal bond length, same for the bending energy and van de waals. However the torsional energy is greater for molecule 4, this is probably due to the double bond being more stable in a 6 membered ring than a 5 membered.
Stereochemistry of Nucleophilic additions to a pyridinium ring (Nad+ analogue)
The first reaction being investigated is the Grignard addition of MeMgI to compound 5 which is a prolinol. The second reaction involves the reaction of aniline with compound 7. The reaction mechanism of the first reaction is shown below.
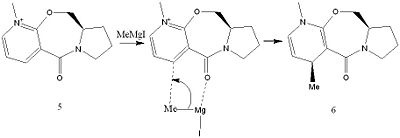
This shows the co ordination of the Mg with the carbonyl oxygen, this is shown the the journal [2] The Me group has to be added to the same face that the oxygen is orientated towards, this explains the stereochemistry. The starting materials were both modelled using ChemBio Draw 3D and the energies minimized as before. For the first reaction it can not be modelled with MeMgI because no atom type can be assigned to Mg and the MM2 will not run. The dihedral angle of the carbonyl ring with respect to the ring was found for compound 5, the lowest energy form had a small dihedral angle in the plane above the ring. This is why the group added is above the ring because the MeMgI group is not large enough for the Me group to be added below the ring. The geometry of the carbon oxygen double bond was changed in the molecules, the greater the dihedral angle the greater the energy. Then the bond was moved to below the molecule the energy was the highest which is why the Me group would not be added under the ring. For compound 7 the C=O group had a small angle in the plane below the ring, however the NHPh group is still added above the ring, this is due to steric hinderance. The lone pairs on oxygen and nitrogen would have a unfavourable interaction so the nucleophilic addition would take place above the ring. Again the C=O angle was varied, the greater the angle the greater the energy and if the C=O was moved above the ring the energy was raised. This model could be improved if the MOPAC/PM6 method was used, this would show the molecular orbitals and would show the orbital interactions to show why the NHPh group is added above the ring because MM2 is just a mechanical method and does not take electronic interactions into account.


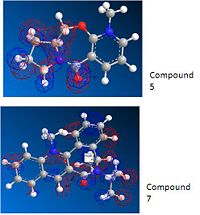
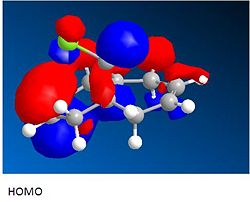
Returning back to this section after having carried out the calculations in the rest of this module there are methods carried out which could improve the analysis of this reaction. The geometry was optimised using MOPAC interface and method PM6, this takes into account electronic effects. The optimised geometry of compound 5 still has a C=O angle above the ring but at a greater angle of 26 degrees. The optimised geometry of compound 7 has the C=O below the ring and again at a greater angle of - 44 degrees. These angles are now optimised with respect to molecular orbitals. The MOs were inspected and the molecular orbital including the C=O is shown below. For compound 5 the orbital can be seen above the plane of the ring which will interact with the Mg and in compound 7 the orbital on the oxygen can be seen below the ring which would would have a steric clash with the approaching nucleophile.
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
Compound 9 and 10 are isomers, one of which is more stable and is an intermediate in the formation of taxol. Compund 9 has a C=O bond above the plane of the ring and 10 has it below. Due to the restricted rotation of this ring they are antropisomers.
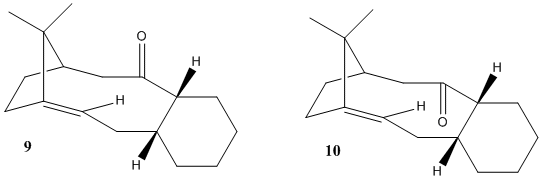
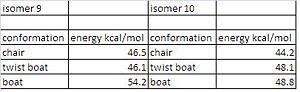
Both isomers were modelled and their energies minimised using MM2 to find the most stable isomer. The cyclohexane ring can have three conformations; chair, boat and twist boat. To find out which isomer was the most stable these atoms had to be manually moved into each configuraton. The chair conformation for cyclohexane on its own is normally the most stable due to torsional and steric strain in the other conformations. When each configuration was found for the cyclohexane the atoms on the brigded alkene group were moved so theres was less steric clash to find the minimun energy. This was quite difficult and many different starting arangements were tried. From the table of energies it can be seen that isomer 10 with the cyclohexane ring in the chair conformation has the lowest energy so it is the most stable. For isomer 9 the energy is lower for the twist boat than the chair, when looking at the models it can be seen that there is greater steric clash between the the cyclohexane ring and the bridging methyl group in the chair conformation. The alkene in the compound reacts slowly, this is because it is a hyperstable alkene as it is next to a bridging group. This journal[3] was used to explain this. Most cycloalkenes have a greater strain energy than the cycloalkenes however for some compounds called hyperstable alkenes this is not the case. This is because when molecules are are added across the double bond for example if H2 was added it would lead to a small decrease in stretching and bending energies energies but a greater increase in torsion and van de waal energies due to the transannular and vicinial hyrdrogen interactions and the angles between the carbon bonds decrease from 124 degrees to 104 degrees (these were measured on ChemBioDraw. So overall the strain energy upon adding a molecule across the double bond would increase the strain energy.
 |
 |
 |
Modelling Using Semi-empirical Molecular Orbital Theory
Regioselective Addition of Dichlorocarbene
Compound 12 was drawn using ChemBio3D and the energy minimised used MM2 then the MOPAC/PM6 method was used to give an approximation for the valence electron molecular wavefunction. This new method is used to take into account effects of electrons. The surface of the molecular wavefunctions can be shown. The one we are focussing on is the HOMO which is associated with electrophilic attack.
 |
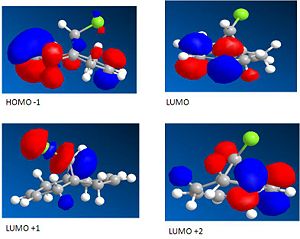 |
 |
From the molecular orbital surface of the HOMO it can be seen that this method does differentiate between the two alkenes. The anti double bond has a much lower electron density than the syn double bond. This suggests that it is the syn double bond is the most nucleophilic and it would take part if an electrophile such as a dichlorocarbene attacked. This is backed up by the journal [4] which explains that the reason behind this is that the σ* Cl-C and the anti π C=C orbital have a favourable interaction which leaves the syn double bond more reactive towards electrophiles. This method takes into account how electrons influence bonds so this can be seen looking at the molecular orbital of the LUMO +1 and HOMO -1.
The density functional approach was then used to find out more information on the C-Cl bond, both structures 12 and 13 were optimised using MM2 then MOPAC/PM6, the Gaussian interface was then used to get an input file to run B3LYP/6-31G(d,p). Then both jobs were sent to SCAN, once they were returned they were opened with Gaussview and the Cl-C and C=C stretching vibrations were found. The vibrations were animated on the molecule and the wavenumbers are given in the table below.

Two C=C stretching frequencies can be found for compound 12 but only the syn double bond is found for compound 13. This is as expected. Both syn C=C stretching frequencies for the compounds are very similar but the Cl-C wavenumber has increased from 12 to 13. This corresponds to an increase in bond strength and a shortening of the Cl-C bond in 13. This corresponds to the analysis above because anti double bond has been removed so the interaction between the σ* Cl-C and the π C=C orbital is not present so the C-Cl bond is stronger. In compound 12 the syn C=C has a larger stretching frequency than the anti, this means the syn C=C bond is stronger which also explains why it would be attacked by an electrophile.
Structure based Mini project using DFT-based Molecular orbital methods
The project chosen was found in a Journal[5] and the reaction scheme is shown, it is a diastereoselective Prins-Friedel-Crafts reaction which was developed for the synthesis of 4-aryltetrahydropyran derivatives. The first reaction involves the reaction of benzaldehyde with a homoallyl alcohol. This reaction was investigated in the journal by using C and H NMR and was found to stereoselective. The F group on the benzaldehyde is an electron withdrawing group which stabilises the oxocarbenium ion which is an intermediate in the reaction. The reason for the stereoselectivity is due to the stability of this intermediate, the ring is in chair conformation and the substituents are in the equatorial position due to axial compression and steric clash - see the reaction mechanism. MM2 could have been used with the substituents in different orientations and the ring could have been put in the boat and twist boat to prove this. This was proved in the journal as the authors used NOE and single crystal Xray analysis. Even though stereoisomers could form for both substituents on the pyran ring I only investiagted the second part of the reaction for the friedel crafts reaction. The journal only gives data for the one isomer because it is stereoselective but I have analysed two isomers to prove that they obtained the correct isomers. The two stereoisomers are shown in the reaction scheme.
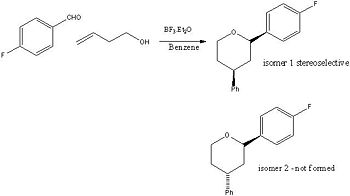 |
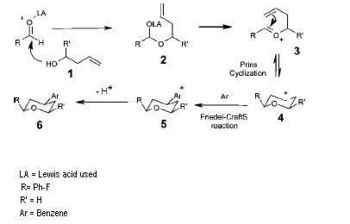 |
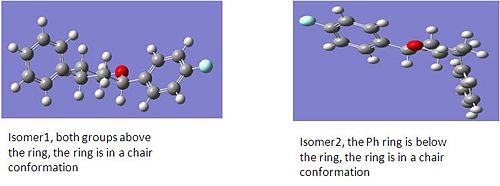 |
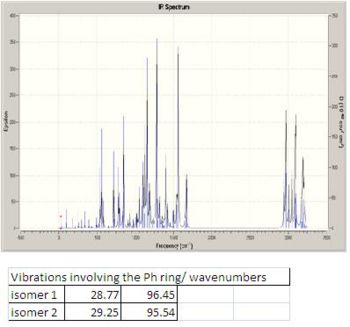
Both isomers were initially drawn in ChemBio 3D and optimised using MM2, as all the elements in the compounds are simple this calculation worked for the molecule. The energies of isomer 1 was 10.2 kcal/mol and for isomer 2 it was 14.8kcal/mol which could suggest one of the reasons that isomer 2 is not formed is because it is higher in energy. However these files were sent to SCAN to optimise their geometries using a PM6 method with a B3LYP procedure and a basis set of 6-31G(d,p). The output file of the optimised geometries was then opened to find the total enegies of each isomer, both isomers had an energy of -833.1 Hartrees which shows the flaws in just using MM2 to optimise the molecules. This then suggests that it is not the energies of the products but the mechanism as explained above which dictates the stereoselectivity. The IR stretches were anaylsed as done previously even though this data was not included in the journal. The spectra for both isomers look very similar, the stretches involving the Ph ring were investigated however there is not much difference in the wavenumbers between both isomers so this is not useful in analysing my compounds.
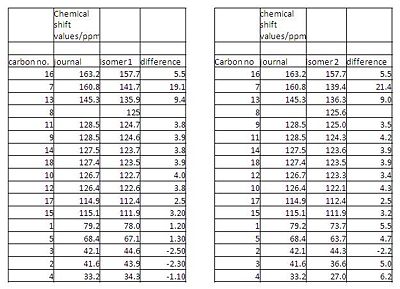
Both isomers were then analysed using 13C NMR. This was done by generating a predicted NMR spectrum of both isomers and comparing to the NMR in the literature. A predicted NMR was generated by optimising the geometries to SCAN using a PM6 method with a B3LYP procedure and a basis set of 6-31G(d,p) then the optimised geomtry outputs were sent back to scan changing the top line of the text file to mpw1pw91/6-31(d,p) NMR scrf(cpcm,solvent=chloroform. The file sent back was opened in GaussView and the NMR was obtained from the results. The predicted NMR of both isomers were compared with the literature NMR, this was difficult for various reasons. The peaks in the journal were not assigned to each atom and there were only 13 shift values whereas in the structure and in the predicted NMR there are 17 shift values. In the literature some peaks are assigned at doublets however due to the low abundance of carbon-13 the probability of there being two carbon 13 atoms adjacent to eachother in the same molecule is very low so these doublet peaks could be wrongly assigned and actually be two peaks very close together. On inspection of the NMR the two different shift values for the peaks were then used. However there were still only 16 shift values, this could be because the NMR calcuation obtained does not account for carbons being in identical environments. Taking all this into account the shifts were assigned to the carbons and the values were compared. The actual values were subtracted from the predicted values for both isomer 1 and 2 and the bar chart shows that the predicted NMR for isomer1 is close to the NMR in the journal and has a lower or similar difference to the predicted values compared with isomer2. This would show that isomer1 was formed. The difference in NMR shift values between the predicted spectrums are also shown, from the table we can see that the largest difference is for atom 7 which is the carbon on the Ph ring which is attached to the pyran ring, this is expected and could be used to differentiate between the isomers. The predicted NMR for isomer1 is published here DOI:10042/to-2942 and for isomer 2 DOI:10042/to-2947 the actual NMR in the journal can be accessed in the extra material of the journal.
 |
 |
 |

The 3J H-H coupling values will not be useful for my compounds because the Ph group which is either above or below the plane of the pyran ring is 3 atoms away from a C with 2 hydrogens, if it was 3 atoms away from the Ph-F group then the dihedral angle between the Hs would be very useful. Optical rotation however could be a very useful technique in distinguishing between the isomers, the predicted OR of each isomer could be predicted by running the optimised geometry with the followin method to SCAN b3lyp/aug-cc-pvdz polar(optrot) scrf(IEFPCM,solvent=chloroform) CPHF=RdFreq this was tried twice however both times the jobs came back incomplete. As the compound has two chiral centres they would rotate light differently so these values could be compared to the experimental value to prove that the correct isomer was formed. This was not reported in the journal. In conclusion the NMR data backs up the mechanistic explaination that only isomer 1 is formed and in the journal many more techniques as mentioned were carried out to prove this.
References
- ↑ Caramella et al J. Am. Chem. Soc., 2002DOI:10.1021/ja016622h
- ↑ Schultz et al J. Org. Chem. 1986,51, 838-841 DOI:10.1021/jo00356a016
- ↑ Camps et all Tetrahedron vol 53 issue 28 Caramella et al J. Am. Chem. Soc., 2002DOI:10.1016/S0040-4020(97)00595-4
- ↑ B. Halton, R. Boese and H. S. Rzepa., J. Chem. Soc., Perkin Trans 2, 1992, 447DOI:10.1039/P29920000447
- ↑ Reddy et al J. Org. Chem., 2009, 74 (6)DOI:10.1021/jo802531h
