MyReport
Introduction to Molecular Modelling 2
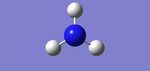
NH3
Molecule Information
Molecule: NH3
Calculation Method: RB3LYP
Basis Set: 6-31G(d.p)
E(RB3LYP): -56.44397188 au
RMS gradient: 0.05399560 au
Point Group: C3V
Optimized N-H Bond Length = 1.01798Å
A literature value for the N-H Bond Length = 1.019Å - Reference
Optimized H-N-H Bond Angle = 105.741°
A literature value for the H-N-H Bond Angle = 109.1° - Reference
Item Table
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES Predicted change in Energy=-5.986268D-10 Optimization completed.
Image of Molecule
3D jmol file
NH3 |
The optimisation file is linked to here
Vibrations
how many modes do you expect from the 3N-6 rule? 6 which modes are degenerate (ie have the same energy)? Modes 5 and 6 which modes are "bending" vibrations and which are "bond stretch" vibrations? Bending - 1, 2, 3 Stretching - 4, 5, 6 which mode is highly symmetric? Mode 4 one mode is known as the "umbrella" mode, which one is this? Mode 1 how many bands would you expect to see in an experimental spectrum of gaseous ammonia? 4
Charges
Charge on N = - 1.125
Charge on H = 0.375
I would expect a negative charge on N, and a positive (but smaller) charge on H because N is more electronegative than H (and there are three H atoms and only one N atom).
H2
Molecule Information
Molecule: H2
Calculation Method: RB3LYP
Basis Set: 6-31G(d.p)
E(RB3LYP): -1.17853936 au
RMS gradient: 0.00000017 au
Point Group: D*H
Optimized H-H Bond Length: 0.74279
A literature value for the H-H Bond Length = 0.74Å - Reference
Item Table
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES Predicted change in Energy=-1.164080D-13 Optimization completed.
3D jmol file
H2 |
The optimisation file is linked to here
Vibrations and Charges
Frequency = 4465.68 cm^-1 (positive)
N2
Molecule Information
Molecule: N2
Calculation Method: RB3LYP
Basis Set: 6-31G(d.p)
E(RB3LYP): -109.52359111 au
RMS gradient: 0.02473091 au
Point Group: D*H
Optimized N-N (Triple) Bond Length: 1.10550
A literature value for the N-N (Triple) Bond Length = 1.10Å - Reference
Item Table
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
Predicted change in Energy=-3.401071D-13
Optimization completed.
3D jmol file
N2 |
The optimisation file is linked to here
Vibrations and Charges
Frequency = 2457.33 cm^-1 (positive)
Haber-Bosch Reaction Energy Calculation
E(NH3) = -56.44397188 au (8 d.p.)
2*E(NH3) = -112.88794376 au (8 d.p.)
E(N2) = -109.52359111 au (8 d.p.)
E(H2) = -1.17853936 au (8 d.p.)
3*E(H2) = -3.53561808 au (8 d.p.)
ΔE = 2*E(NH3)-[E(N2)+3*E(H2)] = 0.17126543 au (8 d.p.)
= 449.657386465 kJ/mol = + 449.66 kJ/mol (2 d.p.)
As ΔE is a positive value, it suggests the conversion of nitrogen and hydrogen gas into ammonia is an endothermic process, and thus that the reactants are more stable than the ammonia product. However, this is not the case - this reaction is known to be weakly exothermic (Reference), which suggests that the energy value for one of the three molecules is incorrect.
My Molecule: S2
Molecule Information
Molecule: S2
Calculation Method: RB3LYP
Basis Set: 6-31G(d.p)
E(RB3LYP): -796.32599779 au
RMS gradient: 0.00000372 au
Point Group: D*H
Optimized S=S Bond Length: 1.92943
A literature value for the S=S Bond Length = 1.89Å - Reference
Item Table
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000006 0.000300 YES Maximum Displacement 0.000011 0.001800 YES RMS Displacement 0.000016 0.001200 YES Predicted change in Energy=-7.077703D-11 Optimization completed.
3D jmol file
S2 |
The optimisation file is linked to here
Vibrations
1 stretch at frequency = 697.03 cm^-1(positive).
Charges
As both atoms in the molecule are of the same element, they have no difference in their electronegativities. This means the molecule has no dipole, and both of the S atoms have a 0 charge.
MO pictures
1 (-88.93663)
This is 1σg, the lowest energy molecular orbital in S2, created from the 1s atomic orbitals. As these AOS are very low in energy they do not have any significant overlap, so the resultant orbital is effectively non-bonding.
2 (-5.96349)
3 (-5.96349)
Molecular orbitals 2 and 3 are the degenerate 1πu and 1πg, because they result from the population of the 2p(x) atomic orbitals on both of the atoms. As the 2p AOs are very small, they are not able to interact with each other, hence the fact that MO-2 has the AOs in-phase (normally giving a bonding interaction), and MO-3 has the AOs out-of-phase (normally giving a higher energy anti bonding interaction) is irrelevant to the MO energies. The electrons occupying these orbitals will not interact at all, so the orbitals are non-bonding.
4 (-5.94945)
5 (-5.94945)
Molecular orbitals 4 and 5 are analogous to molecular orbitals 2 and 3, because they are not large enough to interact. However, they are of a slightly higher energy because they are formed from the 2p(y) AOs, instead of the 2p(x).