MolecularmodellingGMG
Molecular Modelling
Ammonia analysis
Ammonia |
Calculation method: RB3LYP
Basis set: 6-31G(d.p)
RMS gradient norm: 0.00000323 au
Point group: C3V
Final energy: -56.55776873 au
Optimised N-H bond distance: 1.01798
Optimised N-H angle: 105.745°
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000014 0.001800 YES RMS Displacement 0.000009 0.001200 YES
The optimisation file is linked here

Ammonia is not a linear molecule, so the number of vibrational frequencies is given by 3N-6. N=4 so 6 modes are expected. However, due to degeneracy only 4 modes are actually observed.
- The degenerate vibrations at 3580.82 are due to assymetric stretching.
- The vibration at 3461 is due to stretching and is the most symmetric of all the modes.
- The degenerate vibrations at 1693 are due to bending.
- The vibration at 1089.54 is known as the 'umbrella mode'.
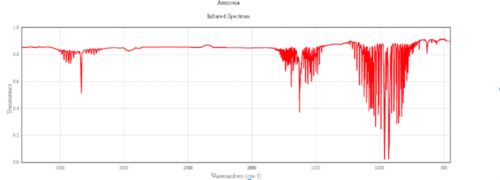
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedAmmonia spectra
Charge on Nitrogen atom: -1.125
Charge on hydrogen atoms: 0.375
It would be expected that Nitrogen to have a negative charge as it a much more electronegative element than Hydrogen.
N2 analysis
Nitrogen |
Calculation method: RB3LYP
Basis set: 6-31G(d.p)
RMS gradient norm: 0.0000060 au
Point group: D∞H
Final energy: -109.52359111 au
N2 triple bond distance 1.092 linear molecule
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
The optimisation file is linked here

H2
Hydrogen |
Calculation method: RB3LYP
Basis set: 6-31G(d.p)
RMS gradient norm: 0.00000017 au
Point group: D∞H
Final energy: -1.17853936 au
H-H bond distance: 0.74279 linear molecule
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
The optimisation file is linked here
Energy of the Haber Process reaction
E(NH3)= -56.55776873
2*E(NH3)= -113.1155375
E(N2)= -109.52359111
E(H2)= -1.17853936
3*E(H2)= -3.53561808
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.05632827 au
ΔE= -147.89 KJ/mol-1
The reaction is exothermic and the product is lower in energy than the reactants. Therefore NH3 is more thermodynamically stable than the gaseous reactants.
F2 analysis
Fluorine |
Calculation method: RB3LYP
Basis set: 6-31G(d.p)
RMS gradient norm: 0.00007365 au
Point group: D∞H
Final energy: -199.49825218 au
F-F bond distance 1.40281
Item Value Threshold Converged? Maximum Force 0.000128 0.000450 YES RMS Force 0.000128 0.000300 YES Maximum Displacement 0.000156 0.001800 YES RMS Displacement 0.000221 0.001200 YES
The optimisation file is linked here

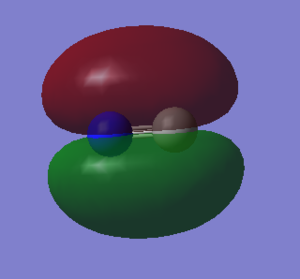
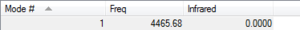
2p orbital combinations
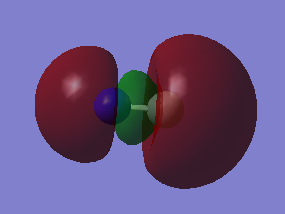
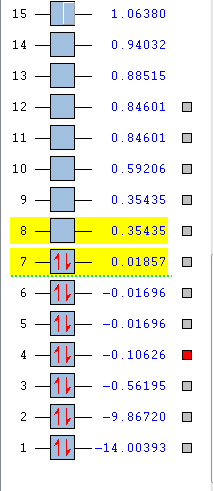
F2 is a diatomic linear molecule. 2 F atoms form a non-polar cavalent bond. The bond order of F2 is one. The p orbitals combine to form 2 pi bonds and a sigma bond. Each orbital is labelled with its bonding or anti bonding character, and the number corresponds to which orbital is represented in the diagram from the list of orbital energies. The HOMO and LUMO are not relatively close in energy compared to the other 2p orbitals.



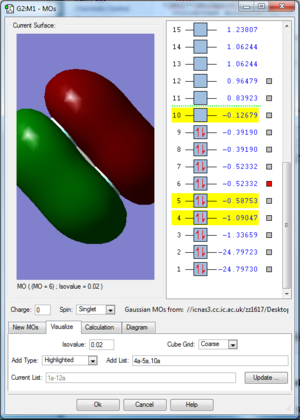
2s orbital combinations
Cyanide analysis
Cyanide |
Calculation method: RB3LYP
Basis set: 6-31G(d.p)
RMS gradient norm: 0.00000704 au
Point group: C∞V
Final energy: -92.82453153 au
Optimised C-N triple bond distance: 1.18409
Item Value Threshold Converged? Maximum Force 0.000012 0.000450 YES RMS Force 0.000012 0.000300 YES Maximum Displacement 0.000005 0.001800 YES RMS Displacement 0.000008 0.001200 YES
The optimisation file is linked here


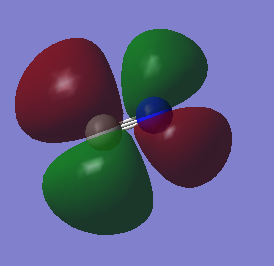
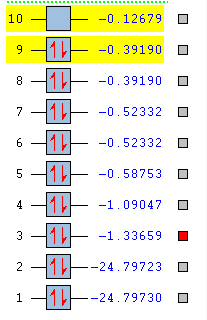
Molecular orbitals of cyanide
2s combinations

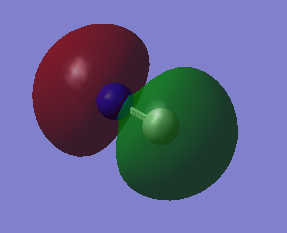
2p combinations
A triple bond is formed between the C and the N atom. The bond order is 2.5. Due to the difference in electronegativity between C and N (nitrogen is the more electronegative atom) the nitrogen atom has higher bonding character and the carbon higher anti bonding character. The number corresponds to which orbital is represented in the diagram from the list of orbital energies