MolecularMechanics:JW1707a
Introduction
Molecular Mechanics is a technique used to model the geometric conformations of a molecule based on the premise that a molecule's conformation is entirely defined its bond properties made up of stretching, bending, torsion, van der waals repulsions and electrostatic interactions. A simple calculation can take all these factors into account and give the geometry of the molecule by trying to minimise the energy across the system.
Part I:
For cyclopentadiene dimerisation:
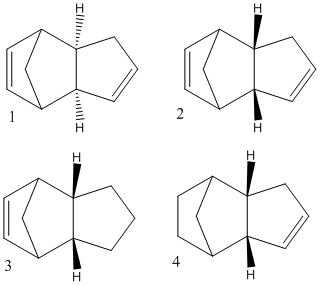
The four molecules on the right were drawn on chemdraw and their energies minimised on ChemiBio3D ultra using the MM2 forcefield, this not only corrected their geometries but also calculated their relative energies in Kcal/mol based on deviation from the normal bond angle/stretch etc. By comparing the the relative energy of the two possible dimers we can conclude which is more thermodynamically stable:
| Isomer | 1 Exo | 2 Endo |
|---|---|---|
| Stretch: | 1.2785 | 1.2556 |
| Bend: | 20.5733 | 20.8429 |
| Stretch-Bend: | -0.8356 | -0.8401 |
| Torsion: | 7.6737 | 9.5183 |
| Non-1,4 VDW: | -1.4179 | -1.5617 |
| 1,4 VDW: | 4.2384 | 4.3461 |
| Dipole/Dipole: | 0.3781 | 0.4502 |
| Total Energy ( kcal/mol): | 31.8885 | 34.0112 |
The values shown indicate that 1, the exo product has a lower energy and hence is the more thermodynamically stable one. However, we know experimentally that 2 the endo product is the major product suggesting that the reaction must be a kinetic one and hence the products are determined by the relative energies of the transition states and not the products.
For the hydrogenation of the endo dimer, a similar calculation was made:
| Isomer | 3 | 4 |
|---|---|---|
| Stretch: | 1.2338 | 1.1029 |
| Bend: | 18.9224 | 14.5263 |
| Stretch-Bend: | -0.7586 | -0.5483 |
| Torsion: | 12.1468 | 12.5095 |
| Non-1,4 VDW: | -1.5102 | -1.0602 |
| 1,4 VDW: | 5.7338 | 4.5000 |
| Dipole/Dipole: | 0.1632 | 0.1407 |
| Total Energy ( kcal/mol): | 35.9313 | 31.1709 |
In the hydrogenation reaction, from the data shown, product 4 is clearly the more stable isomer. The data shows that the major change in energy is due to a change in bending energy, this is possibly due to the ring strain imposed on the norbornane ring by the alkene in product 3, which is relieved by hydrogenation in product 4. A similar effect can be seen by comparing the MM2 forcefield on norbornane and norbornene, which shows that the additional energy of the norbornene molecule is due mainly to extra torsion and bending energies.
Stereochemistry of Nucleophilic additions to a pyridinium ring
The prolinol compound shows two alternative stereochemistries, one of which has the carbonyl group in the same plane as the pyridine ring and one of which has the carbonyl ring coming out in front of the pyridine ring. (Which had minimal energies of Final Energy: 57.4667 kcal/mol, Final Energy: 60.8744 kcal/mol respectively)
Proline conformation 1 |
Proline conformation 2 |
Schultz et al[1]showed that the transition state of this reaction takes the second conformation with the carbonyl sticking forwards. Thus in the transition state where the grignard group bonds to the carbonyl oxygen and forms a 6 membered ring it is with the methyl adding to the pyridine ring from in front, thus the reaction will go almost entirely to the product with the methyl group attached in the up configuration
In the pyridinium molecule, the MMFF94 force field was used to the minimal energy conformations. The methyl group coming out of the 7 membered ring ( which Leleu et al.[2] call "the chiral inducer")was found to force the ring into a specific geometry with the carbonyl group pointing either behind the pyridine ring or in front of it. The repulsion of the incoming amine group to the carbonyl will mean that it is favourable for the amine to add from the opposite face to the carbonyl group. The isomer with the carbonyl group trans to the N-methyl on the pyridine was found to be of significantly lower energy than the isomer with the carbonyl cis to the N-methyl (98.5 kcal/mol vs. 104.2kcal/mol ) as such the main isomer will be the trans isomer so the main product is the one with the amine facing forwards.
NAD+ back |
NAD+ front |
Taxol Precursor Reaction
For the Taxol precursor reaction, a few possible isomers where found. The reason for this is that the way the force field calculation works is monitoring the change in gradient of the energy while moving/stretching and twisting bonds, when a minimum is found where the gradient falls to zero it assumes that the minimal energy conformation has been found. Though sometimes there are localised minima which stops the calculation even though there are lower energies possible. By tweaking the bond angles ( e.g ensuring that the hexane ring was in the chair conformation) to minimise energies, two major conformations were found: one with the carbonyl group facing up and one with the carbonyl group facing down. The one with the carbonyl group facing down was found to have the lower energy. This was then compared to the equivalent molecule with a saturated alkene to try and explain the slow reaction rate of the alkene group.
| Isomer | Carbonyl Up | 2 Carbonyl Down | Carbonyl down with open alkene |
|---|---|---|---|
| Stretch: | 2.6568 | 2.4106 | 2.9282 |
| Bend: | 15.7699 | 11.8461 | 12.9421 |
| Stretch-Bend: | 0.3937 | 0.1974 | 0.4645 |
| Torsion: | 18.2558 | 16.2442 | 19.8711 |
| Non-1,4 VDW: | -1.024 | -1.1261 | -0.4238 |
| 1,4 VDW: | 12.6871 | 12.1919 | 14.4179 |
| Dipole/Dipole: | 0.1451 | 0.262 | 0 |
| Total Energy: kcal/mol | 48.8845 | 42.0261 | 50.2001 |
When the minimisation was repeated using the MMFF94 force field minimisation calcualtion rather than the MM2 the CO down isomer was found to have the lower energy again (60.07 vs 70.54 kcal/mol), which is to be expected. The MMFF94 uses slightly different calculations to determine the force field, so it is expected to find a different figure but the relative energies should show the same isomer to have be more stable. The slow reaction time time for the functionalisation of the alkene can be attributed to the hyperstablised alkene, Maier and Schleyer [3]. proposed the idea and it has since been proven that certain bridgehead olefins do not follow Bredt's rules (which predicts that bridgehead olefins below a certain size will be particularly reactive and unstable). The reason fro this is that in these so called antiBredt olefins, the ring strain of the large rings is in fact made worse by hydrogenating the alkenes. And thus it is not thermodynamically favourable to open the alkene. The calculation above shows the relative energies of the taxol precursor with and without the alkene bond and it is clear that there is significantly higher torsion and van der waals interactions in the saturated ring.
Part II
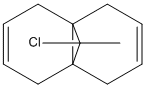
The dialkene compound, has been shown to react selectively with electrophillic reagents, only on the ring with the Cl in it.
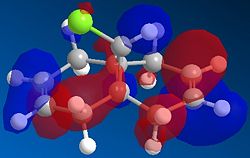
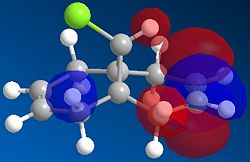
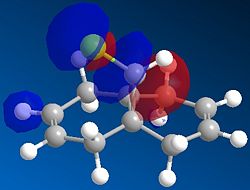
Using modelling techniques, the molecular orbitals responsible for these reactions can be displayed and hence an explanation can be given which may explain the selective reactivity.
It is immediately clear from looking at these orbital diagrams that the main electron density in the HOMO is contained in the alkene below the Cl ( the endo alkene), justifying it's selective reactivity. A closer look at the other orbitals explains this a bit further; there is a strong overlap between the π orbital on the exo alkene seen in the HOMO-1 and the C-Cl σ* antibonding orbital seen in the LUMO+1. As explained by Halton Boeseb and Rzepa [4], this means that the electron density from the exo alkene can be donated into the antibonding C-Cl orbital which will weaken the C-Cl bond. This can then be seen in the vibrational spectra. Comparing the IR frequency associated with the C-Cl in the dialkene compound gives a frequency at 770.83 (cm-1) if we then take away the exo alkene and look at the same vibration it has a frequency of 779.93 cm-1. As seen in the HOMO there remains most of the electron density on the endo alkene which will be free to react.
Part III - Mini Project
Reaction
For the IntraMolecular Simmons-Smith (IMSS) reaction for the synthesis of 5-(Methoxymethoxy)-tricyclo[4.3.0.01,5]nonane the literature[5] shows that both theoretically and experimentally that mainly the endo product is formed in a ratio of 16:1.
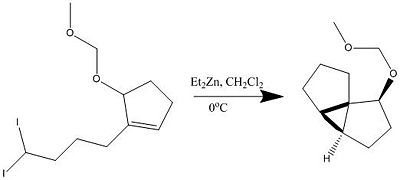
In the following section molecular mechanic techniques will be used to predict the 13C NMR and the IR spectra of both the exo and endo isomers of the molecule reported. This will then be compared with the spectroscopic results reported in the literature and will be able to either confirm that the endo product was really the product achieved. Furthermore the calculation will enable us to assign the 13C NMR which wasn't done in the literature.
GIAO 13C NMR Calculation
Initially the endo conformation was drawn on chemdraw and the geometry optimised on chembio3d using the MM2 forcefield, a few manual tweaks eventually gave a minimum energy of 42.1 kcal/mol. However the calculation result stated that some parameters had to be guessed, so a further energy minimisation calculation was performed with 'MOPAC' which optimised the geometry particularly the methoxymethoxy(MOMO) group was rearranged to maximise the anomeric effect. The geometry was then sent to the SCAN supercomputer to run a Gaussian geometry optimisation before running a GIAO 13C NMR prediction. The same procedure was followed for the exo equivalents and then both calculations were compared with the reported literature values.
Results and Analysis
| Experimental results as published (in ppm) | GIAO calculation for endo product (ppm)[6] | difference(ppm) | GIAO for exo product(ppm)[7] | difference (ppm) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| 96.2 | 91.8524 | 4.3476 | 97.2438 | -1.0438 | ||||||
| 80.1 | 73.3308 | 6.7692 | 86.7093 | -6.6093 | ||||||
| 55.1 | 52.8667 | 2.2333 | 53.395 | 1.705 | ||||||
| 40.6 | 41.863 | -1.263 | 43.5886 | -2.9886 | ||||||
| 28.8 | 29.1332 | -0.3332 | 32.3956 | -3.5956 | ||||||
| 27.2 | 28.9323 | -1.7323 | 29.0912 | -1.8912 | ||||||
| 27 | 27.9613 | -0.9613 | 28.8379 | -1.8379 | ||||||
| 23.8 | 25.7673 | -1.9673 | 27.5989 | -3.7989 | ||||||
| 23.1 | 24.8891 | -1.7891 | 27.4813 | -4.3813 | ||||||
| 22.5 | 24.8891 | -2.3891 | 26.8939 | -4.3939 | ||||||
| 21.8 | 23.9308 | -2.1308 | 24.3542 | -2.5542 | ||||||
| Mean deviation from reported data | 0.07 | 2.85 |
Generally the values of the calculation for the endo molecule came out very similar to the experimental values, the small differences of up to 2-3 ppm are to expected as the calculation isnt flawless. It can also be seen that the values for the exo conformation arent too different, which is to be expected as the only major difference is whichway the MOMO group is facing. The one value that stands out is the value for carbon 9 which has a difference of about 6.7ppm from the experimental values in both the endo and exo conformation. This is already a significant difference, there are three possible explanations for this that I can think of:
- the starting isomer wasnt drawn correctly or the calculations didnt proceed correctly,
- The calculations preceded the best they could but there are other interactions not taken into account by the GIAO caluculation
- Interestingly the experimental value is situated almost exactly half way between the endo and exo isomer values. If somehow the MOMO group is labile allowing the MOMO group to move freely and rapidly from endo to exo conformation then the experimental NMR would show an average of the values. This is highly unlikely looking at the conformation and conditions.
To see if perhaps if a more accurate NMR prediction was possible it was attempted again, once using the CSGT model instead of GIAO calculation and once using the 6-31G+ basis set. This however wasn't succesful as the Gaussview programme doesn't have the reference point for the 6-31G+ basis set and using the CDCl3 reference for the other basis gave results which were up to 10ppm off the experimental data. A further calulation was attempted with another basis set but after several hours of running the calculation was aborted.
Assignment
The data retrieved from the calculation can allow an assignment of the published data.

| Experimental results as published (ppm) | Carbon ( based on numbering in adjacent image) |
| 96.2 | 11 |
| 80.1 | 9 |
| 55.1 | 13 |
| 40.6 | 3 |
| 28.8 | 4 |
| 27.2 | 8 |
| 27 | 6 |
| 23.8 | 5 |
| 23.1 | 2 |
| 22.5 | 1 |
| 21.8 | 7 |
1H NMR
Another method used to distinguish between the two isomers is the coupling constant between vicinal hydrogens. The H on the carbon bonded to the MOMO will have a different dihedral angle with the hydrogens on the adjacent carbon depending on whether the hydrogen is facing into the ring or out of it. The Karplus equation allows us to calculate the coupling constant based on the dihedral angle.
| Angle (degrees) | J Coupling Constant (Hz) | Average value | Experimental Coupling constants as published (Hz) | |
| Exo Molecule: | 35.1 | 5.45 | 3.45 | 4.6 |
| 82.8 | 1.45 | |||
| Endo Molecule | 36.2 | 7.67 | 8.31 | 8 |
| 154.7 | 8.95 |
The data shows that the predicted coupling constants match up with the reported results within a reasonable degree of accuracy. The authors of the paper infact used the coupling constants to prove to distinguish between the exo and endo product and based on the intensity of the peaks calculated their ratio of products to be 16:1 endo to exo. The calculation done using Gaussian optimisation and the Janocchio [8] programme have given a good theoretical basis to this distinction.
IR prediction
Another spectroscopic technique which may be able to distinguish between the two compounds would be IR, although obviously the two isomers have the same functional groups the vibrations may be different when pointing into the ring or out of the ring. Upon completing the calculation for the endo and exo isomers, it was noted that there were two particular vibrations which were significantly different. The C-OMOM stretch had a difference of 4 cm-1, but more significantly the C-H stretch on the chiral Carbon was 2292 cm-1 in the exo molecule ( ) and 3026cm-1 in the endo molecule ( ) where the H was pointing out of the ring. Once these had been corrected by the 8% there was still a 32cm-1 difference. Unfortunately neither of these vibrations were of very high intensity, so they were not reported in the literature and a comparison cant be made with the literature value.
Summary and Conclusion
The intramolecular Simmons-Smith reaction for the sythesis of the tricyclic nonane with a MOMO directing group was found experimentally to produce mainly the endo product. Using theoretical, computional techniques to caluculate the properties of the endo and exo isomers it has been possible to compare the theoretical data with the published experimental results and confirm the results, furthermore it has allowed the carbon shifts from the 13C NMR to be assigned to specific carbon atoms. The most conclusive evidence to back up the published data was by using the dihedral angles from the optimised geometry of the endo and exo isomers respectively which could then be used to calculate the 1H NMR coupling constants, this showed relatively good agreement with the published data. In fact the authors of the paper used the relative coupling constants and the intensity of their peaks to establish the ratio of the two isomers. The 13C NMR data implied that the endo isomer was the more likely product but not conclusivley. Overall the experimental data was more similar to the theoretical results for the endo product, however the key peak from the carbon bonded to the MOMO group was worryingly out by about 7ppm from both the exo and endo calculations, despite attempts to use other calculational methods and different basis sets, a better value was not achieved. Calculations of the IR vibrations were succesful in showing at least two stretches which were significantly different in frequency for the endo and exo products, though the vibrations were not of a very high intensity so they couldn't be compared with the literature values which only reported a few high intensity peaks.
Overall it seems like the calculations back up the experimental data in confirmig that the endo product was the major one. The reason for this reaction's selectivity is due to the geometry enforced on the molecule by the MOMO group during the transition state, the Gaussian programme used isn't well suited for calculating the Transition state energies and conformations so this project was limited to an anaysis of the products.
References and citations
- ↑ A. G. Shultz, L. Flood and J. P. Springer, J. Org. Chemistry, 1986, 51, 838: DOI:10.1021/jo00356a016
- ↑ S. Leleu, C.; Papamicael, F. Marsais, G. Dupas, V.; Levacher, Vincent. Tetrahedron: Asymmetry, 2004, 15, 3919-3928DOI:10.1016/j.tetasy.2004.11.004
- ↑ Maier, W. F.; Schleyer, P.v.R. J. Am. Chem. Soc. 1981, 103, 1891. DOI:10.1021/ja00398a003 and see further: McEwen, A. B.; Schleyer, P.v.R. J. Am. Chem. Soc. 1986, 108, 3951DOI:10.1021/ja00274a016
- ↑ B. Halton, R. Boese and H. S. Rzepa J. Chem. Soc., Perkin Trans. 2, 1992, 447 - 448 DOI:10.1039/P29920000447
- ↑ Intramolecular Simmons-Smith Cyclopropanation. Studies into the Reactivity of Alkyl-Substituted Zinc Carbenoids, Effect of Directing Groups and Synthesis of Bicyclo[n.1.0]alkanes. James A. Bull, André B. Charette,J. Am. Chem. Soc. Article ASAP 2010 DOI:10.1021/ja907504w
- ↑ For more complete NMRdata and optimised geometry of the Endo product DOI:10042/to-3723
- ↑ For more complete NMRdata and optimised geometry of the Exo productDOI:10042/to-3722
- ↑ D. A. Evans, M. J. Bodkin, S. Baker G. Sharman, Magn. Reson. Chem. 2007; 45: 595–600DOI:10.1002/mrc.2016