Modlec4918
Molecules
| Values | |
|---|---|
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Final Energy E(RB3LYP) | -56.55776873 a.u. |
| RMS gradient | 0.00000485 |
| Point group | C3v |
| N-H bond length | 1.018 Ångström |
| H-N-H bond angle | 105.7 degrees |
Text File
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
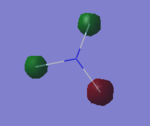
NH3 |
Display Vibrations
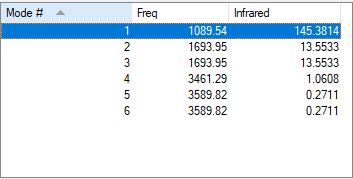
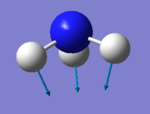
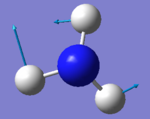
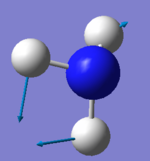
The number of modes of NH3 expected using 3N-6 rule is 6, as N=4. There are two degenerate energy levels at 1693cm-1 and 3589cm-1. On an IR spectrum of gaseous ammonia only two bands at frequencies, 1089cm-1 and 1693cm-1 are seen as only two vibrations result in a change in dipole, due to the unsymmetrical vibrations of the molecule. The 1089cm-1 and 3460cm-1 are symmetric, however 3460cm-1 is highly symmetric as even as the vibrations change the point group remains the same. The bending modes are 1089cm-1 , 1693cm-1 and the stretching 3589cm-1 , 3461cm-1. The bending mode of 1089cm-1 is known as the umbrella mode due the shape of the vibrations made.
| Atom | Charge |
|---|---|
| N | -1.125 |
| H | 0.375 |
The nitrogen in ammonia is electronegative and therefore draws the electrons in the hydroigen bonds towards itself. If the charge on the nitrogen is -1 then the overal charge of the bonded hydrogens is +1 split 3 ways which is +0.333.
Nitrogen
| Values | |
|---|---|
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Final Energy E(RB3LYP) | -109.52412868 a.u. |
| RMS gradient | 0.00000060 a.u. |
| Point group | D*H |
| N-N bond length | 1.11 Ångström |
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES Predicted change in Energy=-3.401072D-13
N2 |
Display Vibrations
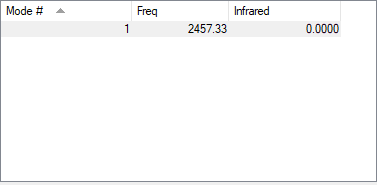
| Wavenumber cm-1 | 2457.3283 |
| Symmetry | SGG |
| Intensity a.u. | 0.0000 |
| Image | 
|
An IR spectrum run on gaseous nitrogen produces no bands as it has only one vibrational mode at 2457cm-1 and this vibrational mode is symmetrical and does not result in a change of dipole moments required to produce a band on IR spectrum.
| Atom | Charge |
|---|---|
| N | 0.000 |
Hydrogen
| Values | |
|---|---|
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Final Energy E(RB3LYP) | - 1.17853936 a.u. |
| RMS gradient | 0.00000017 a.u. |
| Point group | D*H |
| H-H bond length | 0.74 Ångström |
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES Predicted change in Energy=-1.164080D-13
H2 |
Display Vibrations
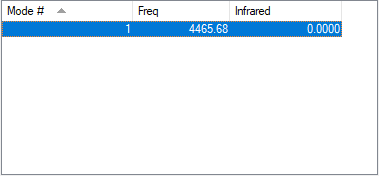
| Wavenumber cm-1 | 4465.6824 |
| Symmetry | SGG |
| Intensity a.u. | 0.0000 |
| Image | 
|
An IR spectrum run on gaseous Hydrogen produces no bands as it has only one vibrational mode at 4465cm-1 and this vibrational mode is symmetrical and does not result in a change of dipole moments required to produce a band on IR spectrum.
| Atom | Charge |
|---|---|
| H | 0.000 |
NF3
| Values | |
|---|---|
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Final Energy E(RB3LYP) | -354.0713058 a.u. |
| RMS gradient | 0.00010256 a.u. |
| Point group | C3v |
| N-F bond length | 1.38 Ångström |
| N-N bond angle | 101.8 degrees |
Item Value Threshold Converged? Maximum Force 0.000164 0.000450 YES RMS Force 0.000108 0.000300 YES Maximum Displacement 0.000612 0.001800 YES RMS Displacement 0.000296 0.001200 YES Predicted change in Energy=-1.274066D-07
N2 |
Display Vibrations
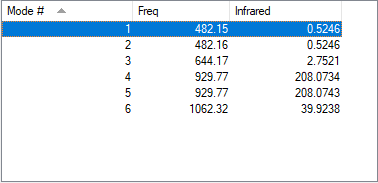
NF3 has 6 vibrational modes. There are two sets of degenerate energy levels, one at 482.15 cm-1 and 929.77cm-1. An IR spectrum run on gaseous NF3 produces two bands, a peak at 929.77cm-1 and 1062.32cm-1, these peaks are visible as the movements result in a change in dipole moments required for a bond vibration to been seen on an IR spectrum.
| Atom | Charge |
|---|---|
| N | 0.660 |
| F | -0.220 |
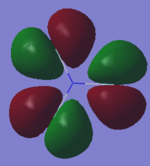
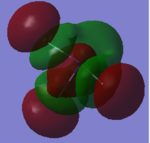
Molecular Orbitals NF3
N2 as a Ligand
| Structural identifier | VEJSOV |
| N-N bond length | 1.12 |
| Link to Structure | [[1]] |
The bond length in the metal ion complex is longer then the experimental. This is for a number of reasons, such as in the computational method the nitrogen is bonded to a metal ion, this will draw electron density away from the nitrogen atom and weaken the bond this is turn lengthens the bond. Another reason is that the computational method measures the bond length when the molecule is in the gaseous phase whereas the experimental is measured in the solid state.
H2 as a Ligand
| Structural identifier | BIDQUB |
| H-H bond length | 0.91 |
| Link to Structure | [[2]] |
Haber-Bosch Process
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]
| Molecule | Energy (a.u) |
|---|---|
| NH3 | -56.5577687 |
| 2*NH3 | -113.1155374 |
| N2 | -109.5241286 |
| H2 | - 1.1785393 |
| 3*H2 | -3.5356180 |
| ΔE | -0.0557907 |
| ΔE (in kJ/mol) | -146.47849401 |
The product is more stable then the reactant as the change in energy is negative.
Marking
Note: All grades and comments are provisional and subject to change until your grades are officially returned via blackboard. Please do not contact anyone about anything to do with the marking of this lab until you have received your grade from blackboard.
Wiki structure and presentation 0.5/1
Is your wiki page clear and easy to follow, with consistent formatting?
YES
Do you effectively use tables, figures and subheadings to communicate your work?
You have used figures and headings however your whole wiki is under the heading "molecules" and you have a subheading called "Text file", these headings don't help the reader.
NH3 0/1
Have you completed the calculation and given a link to the file?
No you have not linked to the file. (I know it is included in the jmol but the labscript asks for a direct link.)
Have you included summary and item tables in your wiki?
YES
Have you included a 3d jmol file or an image of the finished structure?
YES
Have you included the bond lengths and angles asked for?
YES
Have you included the “display vibrations” table?
YES
Have you added a table to your wiki listing the wavenumber and intensity of each vibration?
YES
Did you do the optional extra of adding images of the vibrations?
YES
Have you included answers to the questions about vibrations and charges in the lab script?
YES
N2 and H2 0/0.5
Have you completed the calculations and included all relevant information? (summary, item table, structural information, jmol image, vibrations and charges)
YES - unfortunately you have not linked directly to the log files again.
Crystal structure comparison 0.5/0.5
Have you included a link to a structure from the CCDC that includes a coordinated N2 or H2 molecule?
YES
Have you compared your optimised bond distance to the crystal structure bond distance?
YES
Haber-Bosch reaction energy calculation 0.5/1
Have you correctly calculated the energies asked for? ΔE=2*E(NH3)-[E(N2)+3*E(H2)]
YES
Have you reported your answers to the correct number of decimal places?
No - you should have rounded to 1 or 0.1 kJmol-1, not 8 d.p!
Do your energies have the correct +/- sign?
YES
Have you answered the question, Identify which is more stable the gaseous reactants or the ammonia product?
YES
Your choice of small molecule 2.5/5
Have you completed the calculation and included all relevant information?
YES - however you forgot to link the log file.
Have you added information about MOs and charges on atoms?
YES - good information on the vibrations, and good comment on MO 20, well done! However some of your other MO explanations are not correct, for example MO 5 is the in-phase combination of 2s AOs from all the atoms, not 1s.
Independence 1/1
If you have finished everything else and have spare time in the lab you could: Check one of your results against the literature, or
YES - you have looked up a h2 containing complex in the literature, well done.
Do an extra calculation on another small molecule, or Do some deeper analysis on your results so far