Modelling georgia may green
Molecular modelling lab
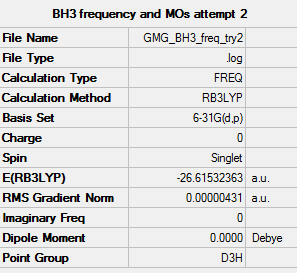
BH3
Summary table:
Item table:
Item Value Threshold Converged?
Maximum Force 0.000012 0.000450 YES
RMS Force 0.000008 0.000300 YES
Maximum Displacement 0.000064 0.001800 YES
RMS Displacement 0.000039 0.001200 YES
Predicted change in Energy=-1.127577D-09
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.1923 -DE/DX = 0.0 !
! R2 R(1,3) 1.1923 -DE/DX = 0.0 !
! R3 R(1,4) 1.1923 -DE/DX = 0.0 !
! A1 A(2,1,3) 120.0058 -DE/DX = 0.0 !
! A2 A(2,1,4) 120.0007 -DE/DX = 0.0 !
! A3 A(3,1,4) 119.9935 -DE/DX = 0.0 !
! D1 D(2,1,4,3) 180.0 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
Low frequency table
Low frequencies --- -2.2544 -1.0770 -0.0055 2.1945 10.2452 10.3017 Low frequencies --- 1162.9859 1213.1756 1213.1783
frequency log file File:GMG BH3 FREQ TRY2.LOG
BH3 |
Molecular orbital diagram of BH3
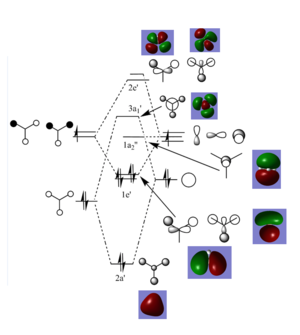
The real LCAOs differ fron from the computed MOs fairly significantly. The computed MOs are much more diffuse than the LCAO compact orbitals. The computed MOs merge and overlap with orbitals of the same phase to a much greater extent. Qualitative MO theory can still give us a clear prediction of which orbitals will overlap to form the MOs through symmetry analysis but cannot give a clear indication of the molecular orbital shapes.
Smf115 (talk) 08:30, 17 May 2018 (BST)Clear inclusion of the MOs in the diagram and both similarities and differences highlighted.
Smf115 (talk) 08:30, 17 May 2018 (BST)The BH3 vibrational section is sadly missing.
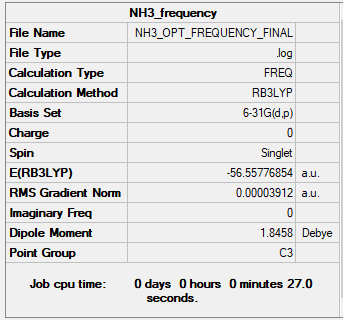
NH3 molecule
File:GEORGIA GREEN OPTIMISATION.LOG
NH3 |
Item Value Threshold Converged?
Maximum Force 0.000006 0.000450 YES
RMS Force 0.000003 0.000300 YES
Maximum Displacement 0.000013 0.001800 YES
RMS Displacement 0.000007 0.001200 YES
Predicted change in Energy=-1.131567D-10
Optimization completed.
-- Stationary point found.
Low frequencies --- -0.0138 -0.0032 -0.0015 7.0783 8.0932 8.0937 Low frequencies --- 1089.3840 1693.9368 1693.9368
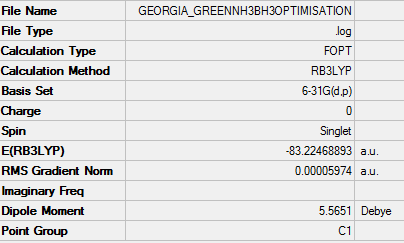
NH3BH3
File:GEORGIA GREENNH3BH3OPTIMISATION.LOG
BH3NH3 |
Item Value Threshold Converged?
Maximum Force 0.000116 0.000450 YES
RMS Force 0.000060 0.000300 YES
Maximum Displacement 0.000583 0.001800 YES
RMS Displacement 0.000346 0.001200 YES
Predicted change in Energy=-1.738666D-07
Optimization completed.
-- Stationary point found.
Dissociation energy calculation
E(NH3)= -56.558
E(BH3)= -25.615
E(NH3BH3)= -83.225
dissociation energy =-83.225-(-56.558+-26.630)
dissociation energy =-0.054A
dissociation energy = -141.7KJ/mol-1
the dissociation of NH3BH3 involved breaking the N-B bond. This is a dative bond, with the nitrogen donating its lone pair into the empty p orbital of BH3. This results in a positive charge of the nitrogen and a positive charge on the boron.
Smf115 (talk) 22:26, 15 May 2018 (BST)The calculation attempt is good. However, the wrong energy value has then been used and a suitable comparison to other bond dissociation energies would be a more relevant comment to make.
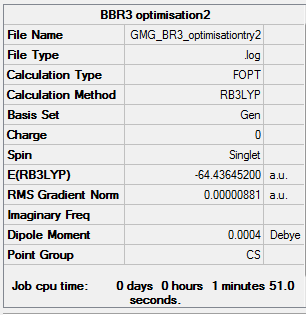
BBr3
File:GMG BR3 OPTIMISATIONTRY2.LOG
BBr3 |
Item Value Threshold Converged?
Maximum Force 0.000024 0.000450 YES
RMS Force 0.000012 0.000300 YES
Maximum Displacement 0.000110 0.001800 YES
RMS Displacement 0.000072 0.001200 YES
Predicted change in Energy=-2.081950D-09
Optimization completed.
-- Stationary point found.
Link to the dspace: DOI:10042/202351
BBR3 has a lower energy and is more stable than BH3 because Br is more electronegative than hydrogen. Therefore the Br atoms can relieve the electron density on the boron.
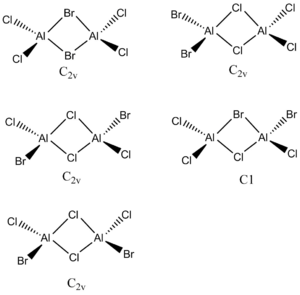
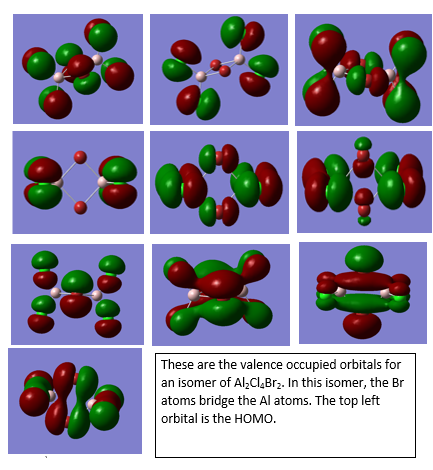
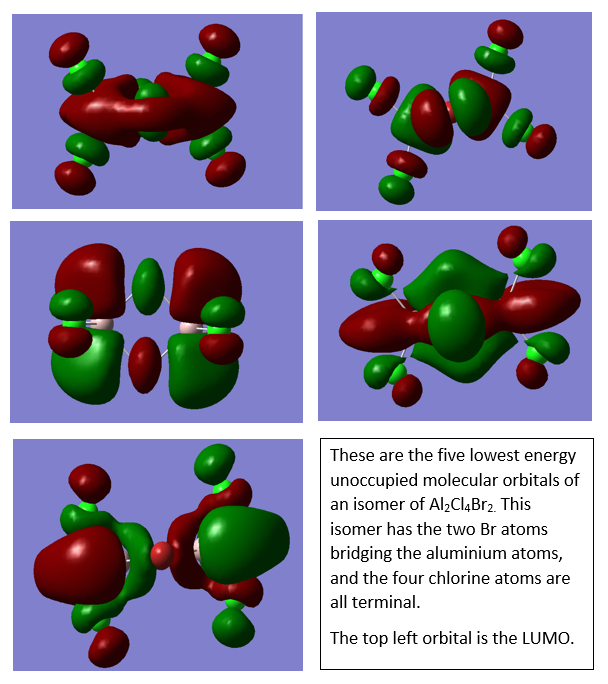
Al2Cl4Br2
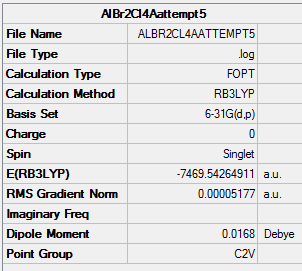
File:ALBR2CL4AATTEMPT5MOANALYSIS.LOG
Isomer A |
Item Value Threshold Converged?
Maximum Force 0.000089 0.000450 YES
RMS Force 0.000055 0.000300 YES
Maximum Displacement 0.001511 0.001800 YES
RMS Displacement 0.000736 0.001200 YES
Predicted change in Energy=-3.596374D-07
Optimization completed.
-- Stationary point found.
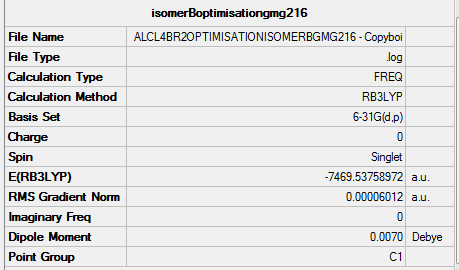
File:ALCL4BR2OPTIMISATIONISOMERBGMG216.LOG
Isomer B |
Item Value Threshold Converged?
Maximum Force 0.000167 0.000450 YES
RMS Force 0.000060 0.000300 YES
Maximum Displacement 0.001614 0.001800 YES
RMS Displacement 0.000719 0.001200 YES
Predicted change in Energy=-3.040027D-07
Optimization completed.
-- Stationary point found.
The energy difference between the two isomers was found to be 13.3KJ/mol-1.
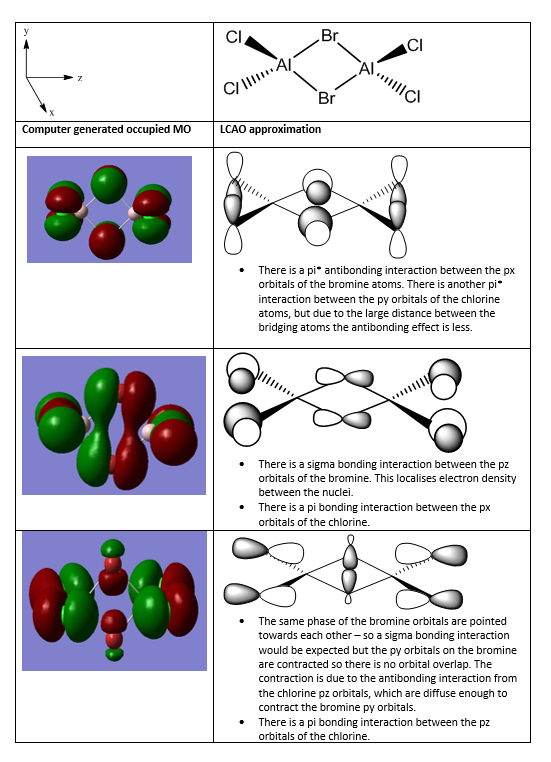
The lower energy isomer is isomer B, when the chlorine atoms are bridging. This could be because chlorine is a more electronegative atom so is more effective at relieving the electron defence on Al. Alternatively, the Br atoms are much larger than the chlorine atoms, so to reduct steric hindrance it is more favourable to have the bromine atoms trans. However, the difference is energy of the two isomers is not large. This could be due to the favourable bonding interactions between the two bromine atoms. The bridging atoms are positioned further apart, but because of the bromine atoms large size and diffuse orbitals, bonding interactions are still possible.
Dissociation of Al2Cl4Br2
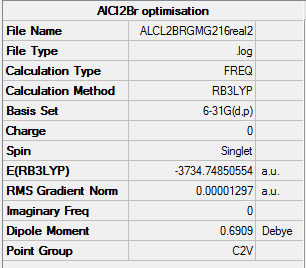
AlClBr |
Item Value Threshold Converged?
Maximum Force 0.000028 0.000450 YES
RMS Force 0.000013 0.000300 YES
Maximum Displacement 0.000083 0.001800 YES
RMS Displacement 0.000048 0.001200 YES
Predicted change in Energy=-3.472099D-09
Optimization completed.
-- Stationary point found.
E(Al2Cl4Br2) = -7469.54
E(AlCl2Br) = -3734.75
Dissociation energy = -7469.54 - (2*-3734.75)
Dissociation energy = -0.041 AU
Dissociation energy = -106.5 KJ/mol-1
The dimer is therefore more stable than the monomers.This is because the monomer AlBrCl2 Br is electron deficient. The central Al atom only has a 6 valence electrons and is sp2 hybridised, meaning there is an empty p orbital on the Al. Because Al can accept a pair of electrons into this orbital to complete its octet, AlBrCl2 is a Lewis acid. In the dimer, the bridging atoms donate their electron density to the aluminium to relieve its electron deficiency.Interestingly, dimerisation happens at low temperatures. One theory to why this is is that the dimer is formed between the LUMO of one molecule of AlBrCl2 and the HOMO of the other overlap to give a sigma bond between the halide and aluminium. However, at high temperature the gap between the HOMO and LUMO increases making this interaction less favourable.
Smf115 (talk) 08:28, 17 May 2018 (BST)Good ideas raised to explain the result in the dissociation energy.
Molecular orbital analysis of Al2Cl4Br2
Smf115 (talk) 08:26, 17 May 2018 (BST)Good descriptions of the key interactions occuring in the MOs. To improve, they could have been more clearly annotated with labelling of extra information such as the nodal planes and some of the mistakes were made with terms such as pi- and sigma-. The wrong conformer was also used however, the LCAOs were correct.
Smf115 (talk) 08:32, 17 May 2018 (BST)Overall a good effort at the report and the project section. However, mistakes were made throughout such as not including the pseudopotential required for your isomers in the second section which resulted in incorrect energies. Despite this good ideas were given throughout for the analysis sections.