ModellingJN1316
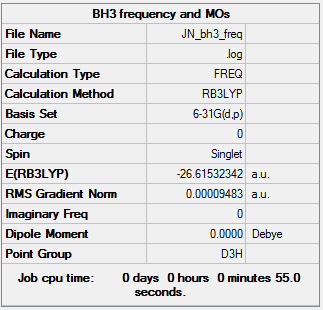
BH3
Method b3lyp Basis 6-31g(d,p)
Item Value Threshold Converged?
Maximum Force 0.000190 0.000450 YES
RMS Force 0.000095 0.000300 YES
Maximum Displacement 0.000747 0.001800 YES
RMS Displacement 0.000374 0.001200 YES
Predicted change in Energy=-2.125549D-07
Optimization completed.
-- Stationary point found.
Low frequencies --- -0.2260 -0.1035 -0.0054 48.0278 49.0875 49.0880 Low frequencies --- 1163.7224 1213.6715 1213.6741
test molecule |
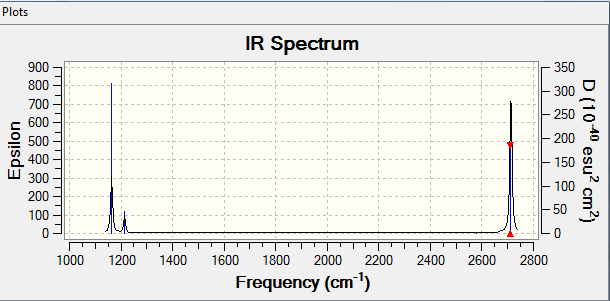
Mode Freq Infrared Active? 1 1163.72 92.4740 Y 2 1213.67 14.0890 Y 3 1213.67 14.0926 Y 4 2579.74 0.00000 N 5 2712.67 126.4185 Y 6 2712.67 126.4089 Y
There are 6 modes but only 3 peaks this is because mode 4 is inactive, 2 and 3 are degenerate and 5 and 6 are degenerate.
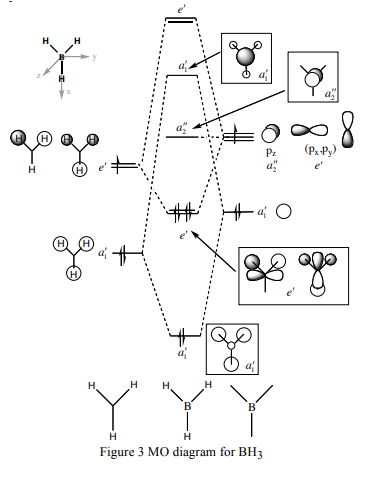
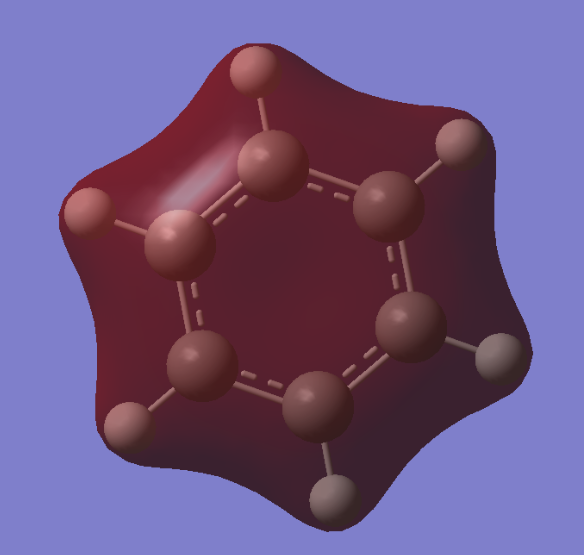
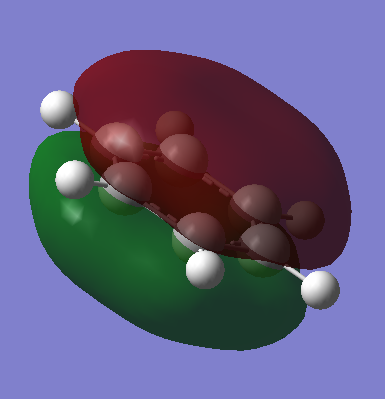
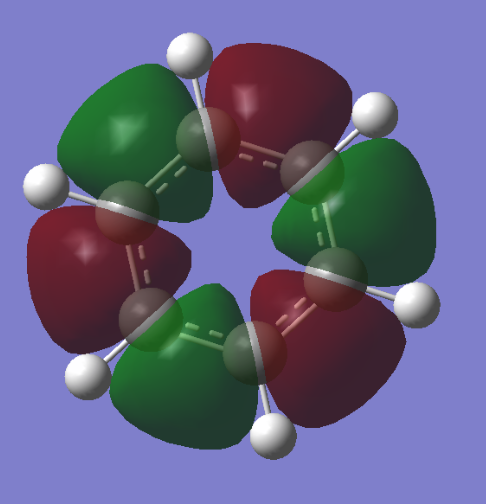
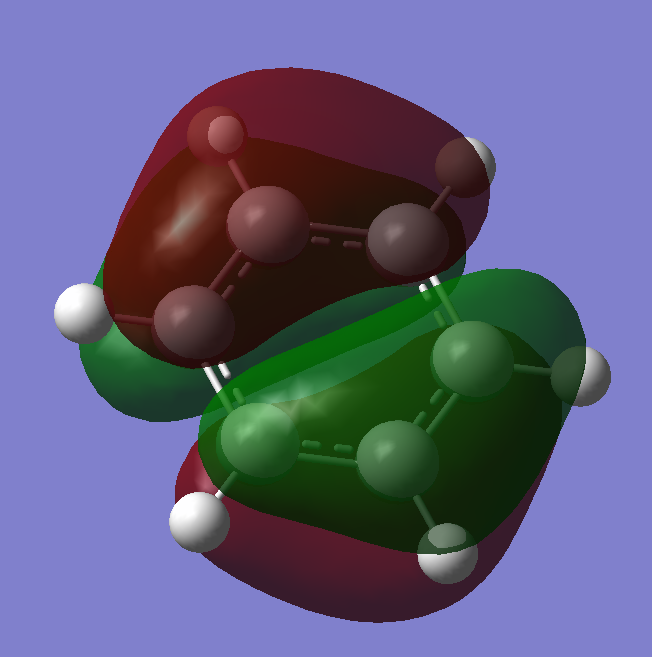
The diagram shows there is little difference between the LCAO MO's and the real MO's (see below) therefore qualitative MO theory is an accurate and useful approximation.
Ng611 (talk) 19:51, 15 May 2018 (BST) Unfortunately you've forgotten to add the real MOs.
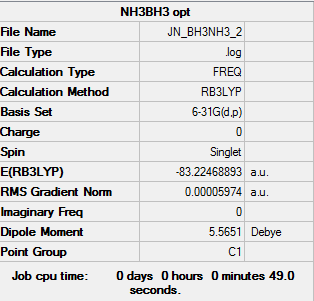
NH3BH3 Item table Item Value Threshold Converged? Maximum Force 0.000021 0.000120 YES RMS Force 0.000014 0.000500 YES Maximum Displacement 0.000601 0.001200 YES RMS Displacement 0.000274 0.001100 YES
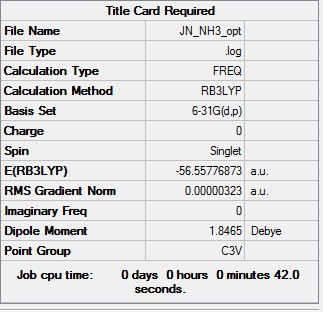
NH3 Item table Item Value Threshold Converged? Maximum Force 0.000002 0.000180 YES RMS Force 0.000003 0.000300 YES Maximum Displacement 0.000014 0.001800 YES RMS Displacement 0.000006 0.001200 YES Low frequencies --- -11.6527 -11.5490 -0.0045 0.0332 0.1512 26.5724 Low frequencies --- 1080.6616 1694.5736 1594.1736
E(NH3) = -56.55776873 a.u. E(BH3) = -26.61532342 a.u. E(NH3BH3) = -83.22468893 a.u. dE = E(NH3BH3) - [E(NH3)+E(BH3)] dE = 0.05159678 a.u. = 135.46 kJ/mol Therefore it is a weak bond for example C-C is 346 kJ/mol
Ng611 (talk) 19:54, 15 May 2018 (BST) Your answers should be to the nearest Kj/mol as this is the maximum level of accuracy that can be achieved with DFT. Your comparison is good but remember to include a literature source for your bond strength (ideally a textbook or a paper).
DSPACE: DOI:10042/202329
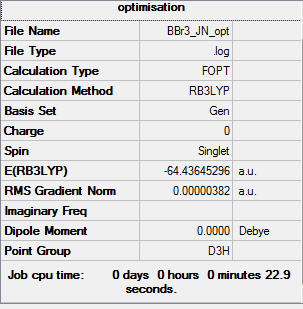
BBr3 Item table Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000007 0.000300 YES Maximum Displacement 0.000036 0.001800 YES RMS Displacement 0.000024 0.001200 YES Low frequencies --- -0.0137 -0.0094 -0.0046 2.5315 2.4315 5.8421 Low frequencies --- 155.9651 156.9651 277.7152
Project Section: Aromatics
test molecule |
test molecule |
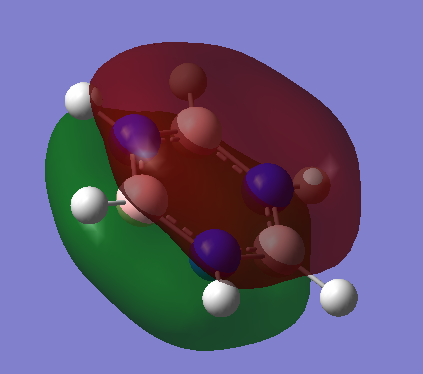
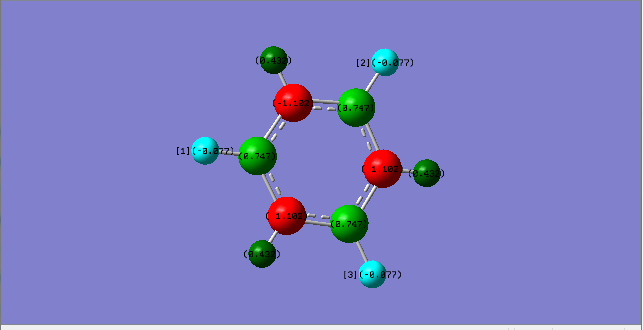
Ng611 (talk) 20:02, 15 May 2018 (BST) Your MO analysis is good. Remember to specify the direction of the p-orbitals (are they aligned with the bond, perpendicular to the plane, etc.) though!
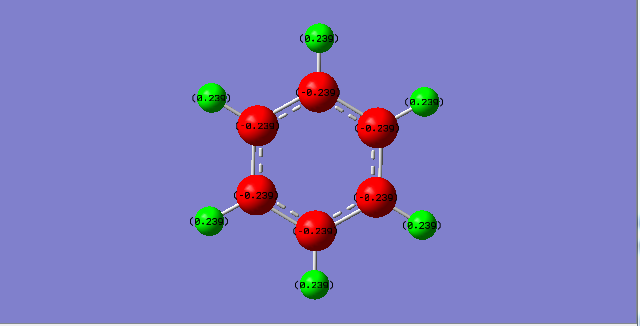
Ng611 (talk) 20:02, 15 May 2018 (BST) Charge analysis also good but you should mention that the sum of the partical charges is 0.
Aromaticity
Aromatic molecules are traditionally defined as flat, cyclic structures with an associated resonance energy that otherwise aliphatic compounds do not have. Aromaticity arises in Benzene, C6H6 as the 6 adjacent p orbitals combine by side on pi bonding to form a delocalised ring of electron density. The resultant system is the quantum mechanical average of all of the canonical forms. Hückel's rule predicts the systems that will experience aromaticity. It states the number of pi electrons in the system must equal 4n+2 where n is a number. The added stability of aromatic systems makes them resistant to electrophilic addition reactions that affect classical alkenes. Modern chemistry has a more nuanced and complex view of aromaticity, it can come in various forms and is not limited to a simple flat system of overlapping pz orbitals. A quasi-aromatic ring can be formed with a 5 atom chain hydrogen bonding to a 6th atom such as hydrogen or lithium. For 50 years scientists have considered the important role of the sigma bond in aromaticity but the subject is still debated. One example of the complexity of aromaticity is the chair conformation of benzene at low temperatures stabilised by its intermolecular interactions. This goes against the classical rule of a planar molecule.
Ng611 (talk) 19:59, 15 May 2018 (BST) You've touched on a few interesting points here, but you need to expand on them somewhat. How exactly are modern views on aromaticity more complex? What is the quantum mechanical understanding of aromaticity? How can we confirm aromaticity in these molecules (NMR, bond enthalpies etc.).
Ng611 (talk) 20:03, 15 May 2018 (BST) This has the makings of a very good report, but needed a bit more time and attention. Your first section in particular was missing key data for structures, MOs etc. The second section was much improved though!