ModRCR16Page
NH3 Molecule
Optimized N-H bond length = 1.01798 Å
Optimized H-N-H bond angle = 105.741°
Charge on the N atom: -1.125
Charge on the H atoms: +0.375
If we consider the formal oxidation numbers usually attributed to ammonia which are -3 for the N atom and +1 for the H atoms, it is obvious that the N-H bonds are not truly ionic, as the covalent character is predominant and cancels out the charges. There still is a lower negative charge on the nitrogen atom because of the difference in electronegativity between N and H.
Calculation Type: OPT+FREQ
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
E(RB3LYP)= -56.55776873 a.u.
Point Group: C3V
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
NH3 Molecule Simulation |
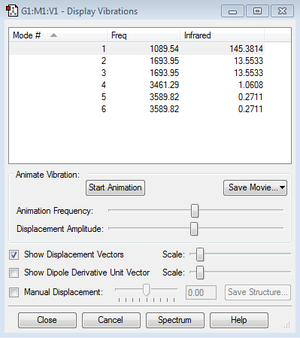
Vibrational Analysis for NH3
6 vibrational modes are expected from the 3N-6 rule, out of which the modes labelled 2 and 3, alongside the modes 5 and 6 are degenerate, as indicated by their frequencies. The modes 1, 2 and 3 represent different bending vibrations whereas 4, 5 and 6 are the stretching vibrations in ammonia. In the 4th vibrational mode, the molecule maintains its C3V symmetry, making this vibration highly symmetrical. The first vibrational mode in the energetic order shows the hydrogen atoms moving together resembling the inversion of an umbrella, which is why it is called the umbrella mode, and this is the basis for many stereochemical properties of organic compounds containing nitrogen. According to the calculated results, the IR spectrum of ammonia should then contain 4 different bands.
H2 Molecule
Optimized bond length: 0.74279 Å
Calculation Type: OPT+FREQ
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
E(RB3LYP)= -1.17853936 a.u.
Point Group: D∞H
The vibrational analysis predicts one band at 4465.68 cm-1
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
N2 Molecule
Optimized bond length: 1.10550 Å
Calculation Type: OPT+FREQ
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
E(RB3LYP)= -109.52412868 a.u.
Point Group: D∞H
Vibrational analysis gives a single band at 2457.33 cm-1
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
Thermodynamic Calculations
E(NH3)= -56.55776873 a.u.
2*E(NH3)= -113.11553746 a.u.
E(N2)= -109.52412868 a.u.
E(H2)= -1.17853936 a.u.
3*E(H2)= -3.53561808 a.u.
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.0557907 a.u.
Converting to kJ/mol, we get ΔE= -146.47849401 kJ/mol
From the estimated thermodynamic values, we see that ammonia is more stable than the two reactants. We see a large difference when comparing this result with the experimentally determined enthalpy of the process, and that shows that the slight inaccuracy of the basis set can propagate a large error when analyzing the macroscopic energetic properties of a molecule.
CH2O Molecule
Charge on the O atom: -0.494
Charge on the H atoms: +0.137
Charge on the C atom: +0.221
Optimized C-O bond length: 1.20676 Å
Optimized C-H bond length: 1.11056 Å
Optimized H-C-H bond angle: 115.220°
Optimized H-C-O bond angle: 122.390°
Comparing the data obtained using Gaussian with the experimental data[1] obtained through X-ray crystallography, we can see a good correlation, showing that the optimization of the structure was successful, but still not perfect. The deviation from the theoretically expected 120° bond angles in the sp2 carbon atom arises due to the fact that oxygen is much larger than the hydrogen atoms, so the structure is distorted so as to minimize electronic repulsions.
Calculation Type: OPT+FREQ
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
E(RB3LYP): -114.50319933 a.u.
RMS Gradient Norm: 0.00006910 a.u.
Dipole Moment: 2.1828 Debye
A noticeable error exists in determining the dipole moment, as the experimentally determined data[2] gives a value of 2.330 Debye. This is most likely due to the difficulty of including all possible intermolecular and intramolecular interactions in the gas phase that can generate a dipole moment.
Point Group: C2V
Item Value Threshold Converged? Maximum Force 0.000182 0.000450 YES RMS Force 0.000080 0.000300 YES Maximum Displacement 0.000231 0.001800 YES RMS Displacement 0.000142 0.001200 YES
CH2O Molecule Simulation |
Vibrational Analysis
CH2O shows 6 non-degenerate vibrations, as expected from using the 3N-6 rule. The results are summarized in the following table:
| Mode Number | Frequency (cm-1) | Infrared Intensity | Type of Vibration |
|---|---|---|---|
| 1 | 1200.65 | 1.5690 | Wagging of CH2 group |
| 2 | 1274.55 | 12.6624 | Rocking of CH2 group |
| 3 | 1554.64 | 6.9116 | Scissoring of CH2 group |
| 4 | 1845.78 | 96.5394 | C=O bond stretching |
| 5 | 2897.28 | 54.5188 | C-H symmetric bond stretching |
| 6 | 2954.04 | 159.3311 | C-H anti-symmetric bond stretching |
Comparison to the determined IR spectrum of formaldehyde[3] shows that the wavenumbers calculated using Gaussian are consistently slightly higher than the real ones and the relative intensities of the bands are slightly different as well.
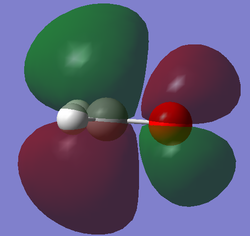
Molecular Orbital Analysis
After computing and estimating the shapes of the molecular orbitals in formaldehyde, the first 12 molecular orbitals have been analyzed and 5 significant ones are represented below:
Study of the Effects of Isotopes in IR Spectroscopy
As we know, the wavenumber of a certain bond stretching vibration depends on the force constant of the bond and the reduced mass of the two atoms that make the bond. Under the ideal harmonic oscillator approximation, this can be described as following:
Taking this into consideration, a Gaussian analysis of the vibrational modes of CHDO, CD2O and 14CH2O molecules was run in order to test this theory. The bond stretching frequencies obtained were compared to the expected frequency values obtained from applying the mentioned formula. Since all the vibrations we analyze are similar to the ones in formaldehyde, we will assume in our calculations that the force constants for isotopically labelled bonds are the same as the natural isotope ones.
As will be shown, the harmonic oscillator approximation shows small errors when predicting the energies of bond vibrations. Nevertheless, its simplicity compared to other models used in IR spectroscopy makes it a reasonable model to use as a preliminary study tool, as isotopic labeling is a common practice in modern-day chemistry.
Computational results for the different isotope-labeled species
1. CHDO
Calculation Type: OPT+FREQ
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
E(RB3LYP): -114.50319917 a.u.
Item Value Threshold Converged? Maximum Force 0.000189 0.000450 YES RMS Force 0.000071 0.000300 YES Maximum Displacement 0.000226 0.001800 YES RMS Displacement 0.000109 0.001200 YES
C-H bond stretch: 2928.06 cm-1
C-D bond stretch: 2159.77 cm-1
The expected value for the C-D bond stretch by taking into consideration the individual C-H stretching that we obtained in the same spectrum would be 2148.61 cm-1, a value which comes into close agreement with the theory.
2. CD2O
Calculation Type: OPT+FREQ
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
E(RB3LYP): -114.50319917 a.u.
Item Value Threshold Converged? Maximum Force 0.000189 0.000450 YES RMS Force 0.000071 0.000300 YES Maximum Displacement 0.000226 0.001800 YES RMS Displacement 0.000109 0.001200 YES
C-D symmetric stretch: 2119.83 cm-1
C-D anti-symmetric stretch: 2206.62 cm-1
The expected values for the symmetric and anti-symmetric bond stretched obtained from the frequencies computed for formaldehyde are 2126.02 cm-1 and 2167.67 cm-1. The values are, again, close to theory, with a slightly larger deviation for the anti-symmetric stretch.
3. 14CH2O
Calculation Type: OPT+FREQ
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
E(RB3LYP): -114.50319917 a.u.
Item Value Threshold Converged?
Maximum Force 0.000189 0.000450 YES
RMS Force 0.000071 0.000300 YES
Maximum Displacement 0.000226 0.001800 YES
RMS Displacement 0.000109 0.001200 YES
C=O stretch: 1769.92 cm-1
C-H symmetric: 2890.25 cm-1
C-H antisymmetric: 2931.43 cm-1
The expected values calculated from the formaldehyde bands are 1768.84 cm-1, 2881.32 cm-1, and 2937.76 cm-1 respectively. As predicted, the values obtained are very close to the theoretical calculations, with only the anti-symmetrical stretch showing a slight deviation from the harmonic oscillator approximation.
References
- ↑ Gurvich, L.V.; Veyts, I. V.; Alcock, C. B., "Thermodynamic Properties of Individual Substances, Fouth Edition", Hemisphere Pub. Co., New York, 1989.DOI:10.1351/pac198961061027
- ↑ R. D. Nelson Jr., D. R. Lide, A. A. Maryott, "Selected Values of electric dipole moments for molecules in the gas phase", NSRDS-NBS10, 1967.
- ↑ T Nakanaga, S Kondo, S Saeki, "Infrared band intensities of formaldehyde and formaldehyde-d2", J. Chem. Phys., 1982, 76(8), 3860.DOI:10.1063/1.443527