ModOne:hhlk2010
Computational Chemistry Lab Course 2010 - Module 1: Structure and spectroscopy (Molecular mechanics/Molecular orbital theory)
The basic techniques of molecular mechanics and semi-empirical molecular orbital methods for structural and spectroscopic evaluations
Modelling using Molecular Mechanics
The Hydrogenation of Cyclopentadiene Dimer
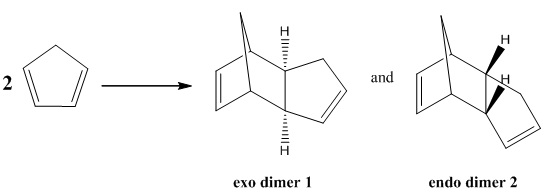
Dimerization of the cyclopentadiene
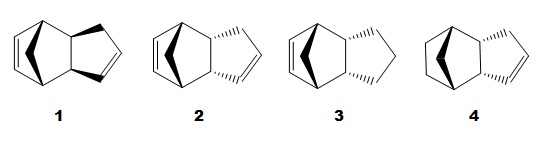
|
|
|
|
||||||||||||
| 1 | 2 | 3 | 4 |
Cyclopentadiene dimerises to produce two different isomeric products, the exo dimer 1 and endo dimer 2. This process is proceeded by a [π4+ π2] cycloaddition reaction.
| Potential function (kcal/mol) | Exo dimer 1 | Endo dimer 2 |
| Stretch | 1.2835 | 1.2670 |
|---|---|---|
| Bend | 20.5793 | 20.8398 |
| Torsion | 7.6711 | 9.5143 |
| VdW | 4.2392 | 4.3288 |
| Total Energy | 31.8836 | 34.0020 |
Table 1: Potential functions of cyclopentadiene dimers
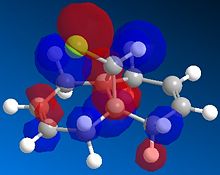
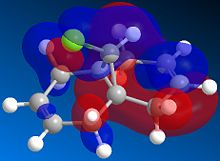
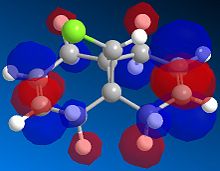
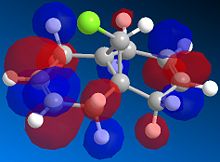
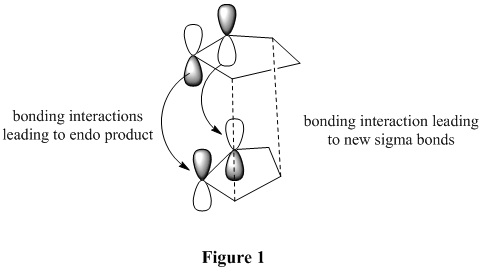
The exo dimer has the lower energy than the endo dimer (see Table 1). The exo dimer 1 is therefore thermodynamically more stable as it is less strained and is preferred to be the major product. However, the endo dimer 2 predominates in the dimerization of cyclopentadiene. This can be explained by the secondary orbitals interactions between two cyclopentadienes. [1] The frontier orbitals in the two components come together for the reaction and the symmetry is correct for bond formation. (see Figure 1) The dashed lines show the orbitals are overlapping to form the new sigma bonds. For the back of the diene, the symmetry of the orbitals is still correct for a bonding interaction. Although this interaction does not lead to any formation of new bonds, it influences the stereochemistry of the product. The endo dimer 2 is favoured because of this favourable interaction between the orbitals.
Furthermore, the endo dimer 2 is a kinetically favourable product which has a lower energy transition state. As the Diels-Alder reaction is irreversible, the endo dimer 2 predominates. [2]
Hydrogenation of the cyclopentadiene dimer
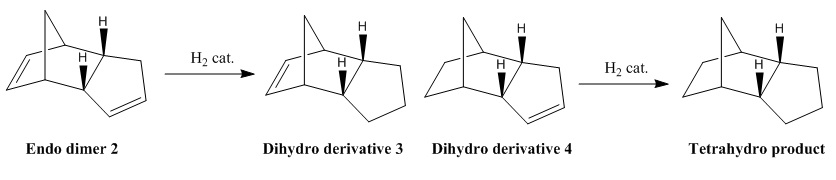
There are two possible dihydro derivatives formed after the hydrogenation of endo dimer 2. (see the above equation) However the reaction only gives the dihydro derivative 4 and the final tetrahydro product is formed after the complete hydrogenation.
| Potential function (kcal/mol) | Dihydro derivative 3 | Dihydro derivative 4 |
| Stretch | 1.2840 | 1.0990 |
|---|---|---|
| Bend | 19.7659 | 14.5123 |
| Torsion | 10.8856 | 12.5074 |
| VdW | 5.6381 | 4.5066 |
| Dipole/Dipole (hydrogen bonding) | 0.1621 | 0.1407 |
| Total Energy | 35.6886 | 31.1681 |
Table 2: Potential functions of the dihydro derivatives
The potential functions of the two dihydro derivatives were carried out and compared in the table above. The dihydro derivative 3 has significantly higher bending strain than the dihydro derivative 4. This is because the C=C bond in the dihydro derivative 4 has a stronger bond strength and a shorter bond length than the C=C bond in the dihydro derivative 3. And the Van der Waal's Force, hydrogen bondings and the total energy of the dihydro derivative 4 are smaller than derivative 3 which show the derivative 4 is thermodynamically more stable. This observation shows that the hydrogenation of the double bond in the dihydro derivative 4 has a faster rate than the hydrogenation of the double bond in the dihydro derivative 3. Thus, The hydrogenation of endo dimer 2 initially gives the dihydro derivative 4 only.
Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+ analogue)
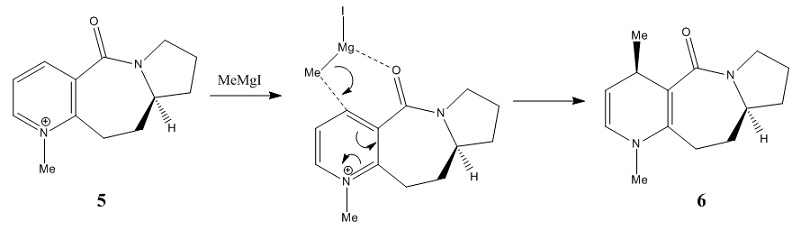
For the reaction of MeMgI with N-methyl pyridoxazepinone 5, the electropositive Mg atom coordinates to the electronegative O atom as shown above. The coordination allows the Me group to form a new bond with the top face of the adjacent C4 position on the pyridinium ring through the nucleophilic addition to generate the highly stereo- and regio- selective product. [3]
| Potential function | Conformation 1
|
Conformation 2
|
Conformation 3
| |||||||||
| Stretch (kcal/mol) | 2.0714 | 3.5909 | 12.3694 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bend (kcal/mol) | 14.2461 | 74.9964 | 131.7160 | |||||||||
| Stretch-Bend (kcal/mol) | 0.1321 | -1.2201 | -2.6610 | |||||||||
| Torsion (kcal/mol) | 5.0418 | 45.6202 | 76.5521 | |||||||||
| Non-1,4 VdW (kcal/mol) | -0.5232 | -2.7505 | 2.1919 | |||||||||
| 1,4 VdW (kcal/mol) | 16.5777 | 27.0996 | 37.7786 | |||||||||
| Charge/Dipole (kcal/mol) | 9.2950 | -6.4481 | -10.7862 | |||||||||
| Dipole/Dipole (kcal/mol) | -4.0156 | -3.0257 | -5.4140 | |||||||||
| Total Energy (kcal/mol) | 42.8254 | 137.8628 | 241.7468 | |||||||||
| Dihedral angle (Degrees) | 9.50 | 94.00 | 12.00 |
Table 3: Different conformations of the active derivative of prolinol 5
Three conformations were carried out and calculated in Table 3. The optimised conformation is then be selected by choosing the lowest total energy of the conformation. Hence, Conformation 1 is the optimised structure with the total energy of 42.8254 kcal/mol. The geometry of N-methyl pyridoxazepinone 5 was optimised by the dihedral angle between the O atom in the carbonyl group and the C4 position of the pyridinium ring. The optimal dihedral angle is 9.5o. As the O atom of the carbonyl group is 9.5o above the plane of the ring, the Me group of MeMgI is directly positioned above the C4 position of the pyridinium ring. Therefore the nucleophilic attack by the MeMgI from the top face of the C4 position is favoured.
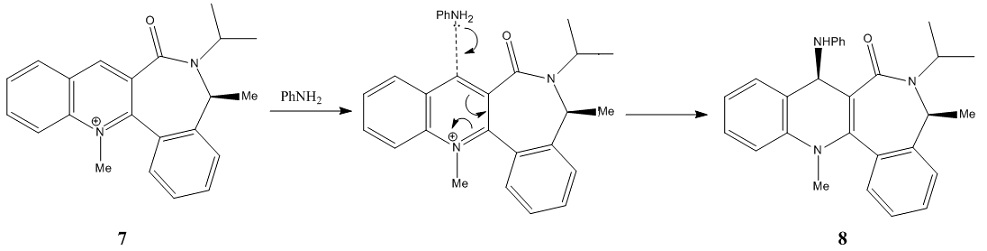
For the reaction of the pyridinium ring of 7 and PhNH2, the amines react on the opposite face with respect to the C=O bond on the pyridinium ring to generate a new NHPh bond and stabilised the positive charge on the N atom. [4]
| Potential function | Conformation 1
|
Conformation 2
|
Conformation 3
| |||||||||
| Stretch (kcal/mol) | 6.1316 | 6.0011 | 4.0740 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bend (kcal/mol) | 18.6590 | 18.1813 | 13.3192 | |||||||||
| Stretch-Bend (kcal/mol) | 0.5477 | 0.5182 | 0.4347 | |||||||||
| Torsion (kcal/mol) | 18.2291 | 19.4720 | 10.1755 | |||||||||
| Non-1,4 VdW (kcal/mol) | 7.2528 | 6.8674 | 4.6637 | |||||||||
| 1,4 VdW (kcal/mol) | 29.7320 | 29.6459 | 29.2413 | |||||||||
| Charge/Dipole (kcal/mol) | 9.4033 | 8.4138 | 9.2805 | |||||||||
| Dipole/Dipole (kcal/mol) | -4.9231 | -4.9188 | -4.8701 | |||||||||
| Total Energy (kcal/mol) | 85.0326 | 84.1807 | 66.3214 | |||||||||
| Dihedral angle (Degrees) | -24.0 | -22.5 | -19.3 |
Table 4: Different conformations of the pyridinium ring of 7
Conformation 3 is the optimised conformation of the pyridinium ring of 7. The determination of the optimised geometry is dependent on the dihefral angle between the O atom of the C=O bond and the C4 position of the pyridinium ring. The optimal dihedral angle in this case is -19.3o which indicates the C=O bond is pointing below the plane of the pyridinium ring.
This result can be used to explain the stereo- and regio- selectivity of the nucleophilic addition on the pyridinium ring of 7. As the O atom of the C=O bond in the ring is 19.3o below the plane of the ring, the nucleophile PhNH2 could not attack from the bottom of the ring due to steric hindrance. Thus, the nucleophile attacks C4 from the top face of the ring instead.
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol.
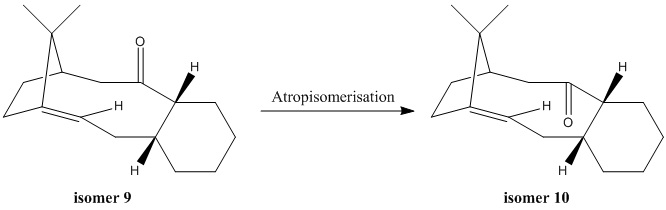
Atropisomers are stereoisomers which prohibit interconversion of isomers and have steric strain hinders rotation about single bonds. The above equation shows the isomer 9 isomerises to a more stable isomer 10.[5]
| Potential function | Isomer 9
|
Isomer 10
| ||||||
| Stretch (kcal/mol) | 2.9989 | 2.5439 | ||||||
|---|---|---|---|---|---|---|---|---|
| Bend (kcal/mol) | 20.8430 | 10.7110 | ||||||
| Stretch-Bend (kcal/mol) | 0.5277 | 0.3242 | ||||||
| Torsion (kcal/mol) | 21.9386 | 19.5945 | ||||||
| Non-1,4 VdW (kcal/mol) | -0.9564 | -1.2398 | ||||||
| 1,4 VdW (kcal/mol) | 14.2907 | 12.5601 | ||||||
| Dipole/Dipole (kcal/mol) | 0.0458 | -0.1811 | ||||||
| Total Energy (kcal/mol) (optimised by MM2 force field) |
59.6883 | 44.3127 | ||||||
| Total Energy (kcal/mol) (optimised by MMFF94 force field) |
82.7052 | 60.5780 |
Table 5: Contributions to energies of Taxol isomers 9 and 10
According to Table 5, isomer 9 has significantly higher bending strain than isomer 10 owing to the different conformations of the cyclohexane ring in two isomers. The cyclohexane ring in isomer 9 is positioned in a strained high energy twist-boat conformation with the C=O carbonyl group pointing up to the plane of the ring. On the other hand, the cyclohexane ring in isomer 10 has a lower energy chair conformation with the C=O carbonyl group pointing down to the plane of the ring.
The total energy of isomer 10 is lower than isomer 9 which indicates that isomer 10 is thermodynamically more stable. And the results of optimised geometry calculations by MM2 force field are matched with the results from MMFF94 force field calculations. Both of them shows isomer 10 has a lower total energy than isomer 9. Hence, isomer 9 isomerises to isomer 10 by atropisomerisation.
Hyperstable olefin explains the reason for some alkene reacts slowly. Hyperstable olefins are used to define the alkenes which are less strained than their parent saturated hydrocarbons. Hyperstable olefins are unreactive because of the greater strain of the parent hydrocarbon which causes resistance to hydrogenation.[6]
Modelling Using Semi-empirical Molecular Orbital Theory.
Regioselective Addition of Dichlorocarbene
Part 1
|
A model of Compound 12 was constructed and shown on the left hand side. Its geometry was optimised by the MM2 force field calculation. The optimal total energy of compound 12 is 17.8965 kcal/mol. The MOPAC/PM6 method was then used to approximate the valence electron molecular wavefunction. Ultimately, the Density-functional B3LYP/6-31G method was applied to obtain a more accurate geometry prediction of compound 12. |
The reaction of Compound 12 with dichlorocarbene is an electrophilic addition. The HOMO and LUMO orbitals control the reactivity of compound 12, the regioselectivity of this reaction is explained by the MO diagrams as the following.
| HOMO-1 | HOMO | LUMO | LUMO+1 | LUMO+2 |
Table 6: Molecular orbitals of Compound 12
The HOMO orbital indicates that there is higher electron density at the syn-alkene than the anti-alkene. (see Table 6)Thus the syn-alkene is more nucleophilic. The electrophile attacks the electron rich syn-alkene as the reaction with dichlorocarbene is an electrophilic addition. On the other hand, the HOMO-1 orbital has higher electon density at the anti-alkene which has been proved by the MO calculations using the PM3 method for MOPAC calculation.[7]
Furthermore, LUMO and LUMO+2 orbitals are the pi* orbitals and the LUMO+1 orbital is the C-Cl sigma* orbital. The orbital mixing between the anti-alkene orbital and another higher energy sigma* orbital can take place to stabilise the two orbitals, hence lowering the energy of both of them.
Part 2
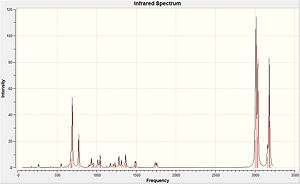
Two molecules are examined and compared to calculate the influence of the Cl-C bond on the vibrational frequencies of this molecule, Compound 12. They are the Compound 12 which contains a double bond anti to the Cl-C bond and a hydrogenated version where this anti double bond is replaced by a C-C single bond.
| Molecule | Cl-C stretch/cm-1 | Cl-C stretch/cm-1 (lit. value)[8] |
syn C=C stretch/cm-1 | syn C=C stretch/cm-1 (lit. value)[9] |
anti C=C stretch/cm-1 | |||
Compound 12
|
770.871 | 780 | 1757.37 | 1620-1680 | 1737.14 | |||
|---|---|---|---|---|---|---|---|---|
Hydrogenated Compound 12
|
780.067 | 780 | 1753.75 | 1620-1680 | --- |
Table 7: Infrared stretching frequencies of Compound 12 and its hydrogenated version.
 |
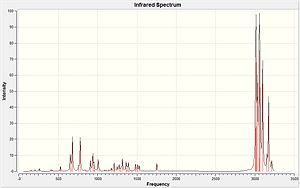 |
According to Table 7, the Cl-C bonds stretching in two molecules are both close to the literature values. But the C=C stretching frequencies are slightly higher than the literature values. To compare the infrared frequencies of the syn- and anti- C=C in Compound 12, the syn- C=C bond has a higher frequency which shows a higher bond energy and hence more reactive. The strength of the C=C bond is dependent on the sigma and pi bonds where electrophilic addition reaction only attacks the pi bond. And the sigma effect of the C-Cl bond on the alkene withdraws the electron density from the sigma bond of the anti-alkene which makes it weaker than the syn-alkene.
Structure based Mini project using DFT-based Molecular orbital methods
Investigating the regioselectivity of the Baeyer-Villiger reaction
The Baeyer-Villiger reaction, which also names as Baeyer-Villiger oxidation, is an organic reaction in which a ketone is oxidized to an ester, effectively inserting an oxygen atom between the carbonyl group and the alpha-carbon. It is carried out using mCPBA. There are two possible regioisomeric products for unsymmetrical ketones. In this section, the synthesis of the kainic acid is chosen to investigate the regioselectivity of the Baeyer-Villiger reaction. The highly regioselective Baeyer-Villiger reaction was a key step during a total synthesis of the natural product - kainic acid. The 13C data of the compounds will be predicted using the GIAO method and compared with the literature values. The regioselectivity of the reaction will soon be explained too.
Mechanism
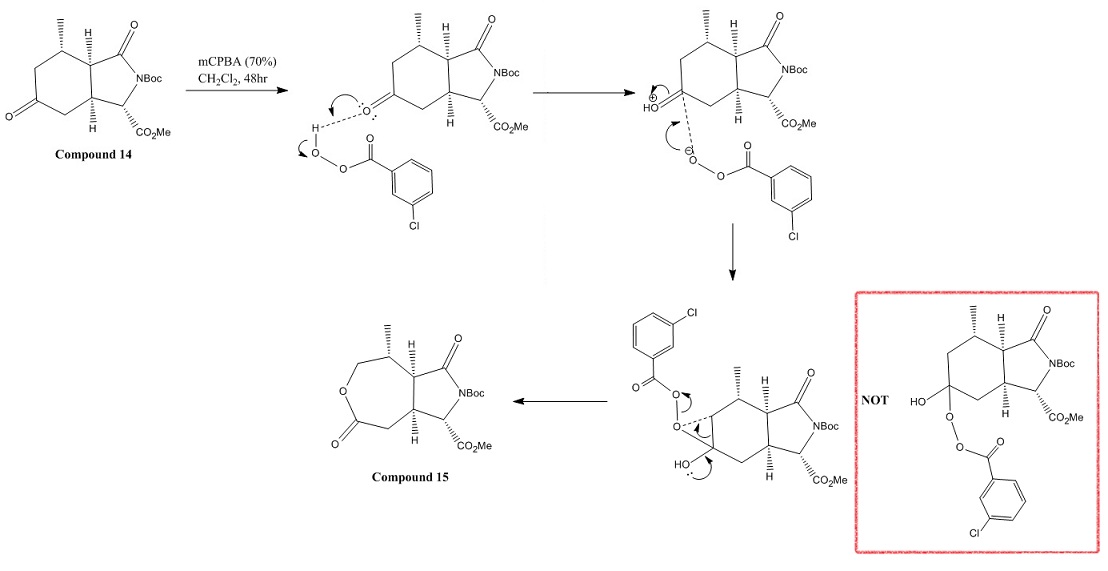
The conversion of compound 14 into 15 is shown as the following:
Contributions to Energies of Compound 14 & 15
|
|
||||||
| Compound 14 | Compound 15 |
| Potential function (kcal/mol) | Compound 14 | Compound 15 |
| Stretch | 2.3574 | 2.7327 |
|---|---|---|
| Bend | 18.3459 | 23.2907 |
| Torsion | 6.7647 | 2.7063 |
| VdW | 13.9141 | 16.4687 |
| Dipole/Dipole (hydrogen bonding) | 17.0269 | 21.6271 |
| Total Energy | 53.7619 | 63.5527 |
Table 8: Potential functions of Compound 14 & 15
Comparing the contributions to energies of compound 14 & 15, Compound 14 has a lower bending strain than Compound 15. This result indicates that Compound 15 experienced higher bond and angle strains by the extra C-O bond. In addition, Compound 15 has higher energies in the Van der Waal's Forces and hydrogen bondings than Compound 14. This is because there is a new C-O bond contributes to the hydrogen bondings. Hence, the total energy of Compound 15 is higher. (see Table 8)
Moreover, Compound 14 contains bulky Boc group and ester group in its 5 member-ring. When Compound 14 reacted with the bulky reagent mCPBA, mCPBA attacked and positioned above the C=O group in the 6 member-ring instead of below it due to the steric hindrance. (see the Mechanism shown above) Therefore, the Baeyer-Villiger reaction is highly regioselective due to the steric hindrance in the structure of the molecules.
13C NMR Spectra Analysis
| Carbon | Chemical shift/ppm | Chemical shift/ppm (lit. value)[10] |
| 8 | 176.149 | 207.95 |
|---|---|---|
| 17 | 171.582 | 172.75 |
| 18 | 170.959 | 170.34 |
| 19 | 166.689 | 149.41 |
| 6 | 161.619 | 84.08 |
| 3 | 158.800 | 62.65 |
| 1 | 158.521 | 52.73 |
| 5 | 148.151 | 47.09 |
| 25 | 148.151 | 44.97 |
| 9 | 131.019 | 42.05 |
| 16 | 115.239 | 34.54 |
| 20 | 53.0207 | 29.34 |
| 22 | 34.5356 | 27.78 |
| 10 | 27.9982 | 20.30 |
Table 9: 13C NMR data of Compound 14
| Carbon | Chemical shift/ppm | Chemical shift/ppm (lit. value)[11] |
| 17 | 170.579 | 171.5 |
|---|---|---|
| 16 | 157.236 | 171.3 |
| 4 | 164.198 | 169.7 |
| 5 | 161.159 | 149.2 |
| 7 | 159.031 | 84.4 |
| 6 | 144.748 | 68.9 |
| 24 | 136.087 | 63.0 |
| 8 | 131.681 | 52.9 |
| 1 | 124.084 | 48.7 |
| 15 | 106.306 | 36.5 |
| 19 | 46.7426 | 32.5 |
| 21 | 27.7493 | 29.8 |
| 3 | 24.4065 | 27.8 |
| 9 | 22.5831 | 15.5 |
Table 10: 13C NMR data of Compound 15
The GIAO calculations are not completely correlated to the literature values. However, The trend of the chemical shifts changing is the same among the experimental data and the calculations results. The chemical shifts of the carbons were decreased after the Baeyer-Villiger reaction of compound 14.
IR Spectra Analysis
| Molecule | C=O stretch/cm-1 | C=O stretch/cm-1 (lit. value)[12] |
| Compound 14 | 1870.64 1827.28 1823.89 1797.06 |
1789 1746 1729 1713 |
|---|
Table 11: Infrared stretching frequencies of Compound 14
| Molecule | C=O stretch/cm-1 | C=O stretch/cm-1 (lit. value)[13] |
C-O stretch/cm-1 | C-O stretch/cm-1 (lit. value) |
| Compound 15 | 1866.88 1850.08 1831.95 1794.82 |
1780 1755 1748 1723 |
1271.78-1306.68 | 1100-1300 |
|---|
Table 12: Infrared stretching frequencies of Compound 15
 |
 |
The IR calculations of both compounds can successfully correlate to the experimental data. (see Table 11 & 12) For Compound 15, there is a C-O stretching at 1271.78-1306.68 cm-1 indicates the ester group in the molecule.
References
- ↑ Clayden, Greeves, Warren and Wothers, Organic Chemistry, 2001, p916-917.
- ↑ J. Sauer, R. Sustmann, Mechanistic Aspects of Diels-Alder Reactions, 1980,19, 779: DOI:10.1002/anie.198007791
- ↑ A. G. Shultz, L. Flood and J. P. Springer, J. Org. Chemistry, 1986,51, 838: DOI:10.1021/jo00356a016
- ↑ S. Leleu, C.; Papamicael, F. Marsais, G. Dupas, V.; Levacher, Vincent., Tetrahedron: Asymmetry, 2004,15, 3919-3928: DOI:10.1016/j.tetasy.2004.11.004
- ↑ S. W. Elmore and L. Paquette, Tetrahedron Letters, 1991, 319; DOI:10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0
- ↑ W.F.Maier, P.v.R. Schleyer, J. Am. Chem. Soc., 1981, 103, 1891: DOI:10.1021/ja00398a003
- ↑ B. Halton, R. Boese and H. S. Rzepa., J. Chem. Soc., Perkin Trans 2, 1992, 447: DOI:10.1039/P29920000447
- ↑ G. Socrates, Infrared and Raman Characteristic Group Frequencies, 3rd Edition, 2001, p.65
- ↑ J. Coates, Interpretation of Infrared Spectra, A Practical Approach, Encyclopedia of Analytical Chemistry, John Wiley & Sons Ltd, Chichester, 2000
- ↑ J. Clayden, C. J. Menet and K. Tchabanenko Synthesis of (−)-kainic acid using chiral lithium amides in an asymmetric dearomatizing cyclization , 2002,58, 4727-4733: DOI:10.1016/S0040-4020(02)00379-4
- ↑ J. Clayden, C. J. Menet and K. Tchabanenko Synthesis of (−)-kainic acid using chiral lithium amides in an asymmetric dearomatizing cyclization , 2002,58, 4727-4733: DOI:10.1016/S0040-4020(02)00379-4
- ↑ J. Clayden, C. J. Menet and K. Tchabanenko Synthesis of (−)-kainic acid using chiral lithium amides in an asymmetric dearomatizing cyclization , 2002,58, 4727-4733: DOI:10.1016/S0040-4020(02)00379-4
- ↑ J. Clayden, C. J. Menet and K. Tchabanenko Synthesis of (−)-kainic acid using chiral lithium amides in an asymmetric dearomatizing cyclization , 2002,58, 4727-4733: DOI:10.1016/S0040-4020(02)00379-4