Mod:Hunt Research Group/option4
Application of Bent's Rule to Nonbonding Interactions
Project
Hydrogen and halogen bonding are ubiquitous in nature and have in recent years have been extensively used in material science. These special nonbonding interactions (along with others) may also be classified as Lewis acid-Lewis base interactions. Lewis acid-Lewis base interactions have been shown to lead to an electron charge redistribution in accordance with Bent's rule. Bent's rule states that "atomic s-character tends to concentrate in orbitals that are directed toward electropositive groups and p character tends to concentrate in orbitals that are directed toward electronegative groups" and is routinely used in both organic and inorganic chemistry to explain deviations in chemical structures.
In this project you will investigate, whether or not, Bent's rule is observed for a series of F3CCl H-bond or halogen bond complexes with H3O+, OCH2, Cl- and OH-. F3CCl is chosen as the Cl centre may act as both the Lewis acid or the Lewis base when interacting with these molecular and ionic species. If Bent's rule is observed there should be a shortening or lenthgening of the C-Cl bond length upon formation of nonbonded interactions. This is confirmed by a change in the s-character in the C-orbital in the C-Cl bond.
For this project you must
1. Use the MP2 level of theory
2. Use the 6-311++g(d,p)full electron basis set for all atoms
3. Report all energies in kJ/mol, unless requested not to.
4. Optimise, carry out a frequency analysis and carry out an NBO analysis of CF3Cl.
5. Optimise and carry out a frequency analysis on the other four species (H3O+, OCH2, Cl- and OH-) to confirm they are fully optimised.
6. Optimise, carry out a frequency analysis, compute the MOs and carry out an NBO analysis of the following complexes; CF3Cl•••H3O+, CF3Cl•••OCH2, CF3Cl•••Cl- and CF3Cl•••OH-. Note in your report the type of interaction (i.e. Hydrogen or halogen bonding) that occurs for each complex. Hint: Three complexes should have a C-Cl•••A angle that is ~linear. The fourth complex will be ~90 degrees
7. Report details (i.e. optimised structures, convergence criteria and several low frequencies) to indicate the CF3Cl complexes are at a minimum on the potential energy surface.
8. Report the energies for the CF3Cl complexes and the individual species (in a.u.).
9. Report the key 'bonds' and 'angle' (between the species) for the complexes and uncomplexed CF3Cl, as well as the binding energy of the complexes.
10. Compare and discuss the observables in point (9) paying attention to the type of interacting species and draw initial conclusions on (a) the type of interaction and (b) the change in s-character in the C-orbital of the C-Cl bond, applying Bent's rule.
11. Report key nbo data for the C-Cl bond, as was the NPA charges for the C and Cl atoms, to support your conclusions from point (10). Hint: Note the nA → σCCl* interaction energy
12. Provide a discussion on the data from point (11).
12. Visualise all the occupied non-core MOs of the complexes. You do not need to reproduce them all.
13. In your wiki report the LUMO and HOMO for each complex and a selection of several other MOs that help (highly bonding to highly antibonding) describe the interactions occuring upon complexation. Can these be related to the NBO analysis?
Introduction
Hydrogen and halogen bonding are ubiquitous in nature and have in recent years have been extensively used in material science. Several nonbonding interactions (along with others) may also be classified as Lewis acid-Lewis base interactions. This poses an interesting question; do H-bonds and halogen bonds follow Bent's rule like Lewis acid-Lewis base interactions? In this project this question is answered using computational chemistry methods to study a series of F3CCl H-bond or halogen bond complexes with H3O+, OCH2, Cl- and OH-. F3CCl is chosen as the Cl centre may act as both the Lewis acid or the Lewis base when interacting with these molecular and ionic species due to the anisotropic charge distribution around the Cl atom in C3FCl. If Bent's rule is observed there should be a shortening or lenthgening of the C-Cl bond length upon formation of nonbonded interactions as well as a change in the s-character in the C-orbital in the C-Cl bond. This is to be observed via Natural Bond Orbital (NBO) and molecular orbital (MO) analysis.
Geometry Optimization and Frequency Calculation outputs
Geometry optimisations and frequency calculations were carried out at the MP2/6-31++G(d,p) level.
The tabulated data below (Tables 1-4) is taken from optimized geometry and frequency output files corresponding to the CF3Cl•••H3O+, CF3Cl•••OCH2, CF3Cl•••Cl- and CF3Cl•••OH- complexes. This data confirms the presence of minimum structures on the potential energy surface for each complex.
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
From the geometry optimisations it is observed (geometric criteria) that the CF3Cl•••H3O+ interaction is a hydrogen bond, while the three remaining complexes form halogen or charged halogen bonds (charged species).
Energies of Complexes and Individual Species
A summary of the computed energies of the complexes and individual species is provided in Table 5.
| Species/Complex | Energy (a.u) |
|---|---|
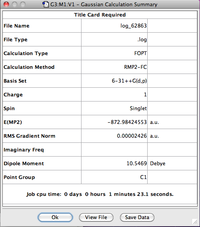
| CF3Cl•••H3O+ | -872.98424553 |
| CF3Cl•••OCH2 | -910.65777058 |
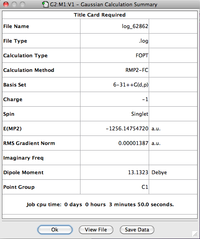
| CF3Cl•••Cl- | -1256.1475472 |
| CF3Cl•••OH- | -872.09240635 |
| CF3Cl | -796.46130200 |
| H3O+ | -76.50588400 |
| OCH2 | -114.19297686 |
| Cl- | -459.539660881 |
| OH- | -75.602656820 |
The energies above could be incorporated into the Tables in the section above.
Geometric and Energetic Properties
| Complex | Cl•••A(B) | C-Cl | C-Cl•••B(A) | Ebind (kJ/mol) |
|---|---|---|---|---|
| CF3Cl•••H3O+ | 2.084 | 1.779 | 92.771 | -44.79 |
| CF3Cl•••OCH2 | 3.038 | 1.735 | 179.975 | -9.17 |
| CF3Cl•••Cl- | 3.087 | 1.718 | 179.962 | -39.64 |
| CF3Cl•••OH- | 2.423 | 1.736 | 176.112 | -74.69 |
A summary of key geometical and energetic parameters for the CF3Cl complexes is presented in Table 6. The Chlorine-H/Hal (Lewis Acid/Lewis Base) bond distances and C-Cl bond length are given in Å and the C-Cl•••B(A) angle in degrees.
For the CF3Cl•••H3O+ complex in which CF3Cl behaves as the Lewis base (H-bonding) the C-Cl•••B(A) angle is observed to be 92.77. In comparision the C-Cl•••B(A) angle is observed to be approximately 180 for the other complexes in which CF3Cl behaves as the Lewis acid (Halogen-bonding). This suggests the anisotropy of the electron charge distribution around the chlorine atom.
Might be worthwhile getting the students to look for examples of the anisotropy playing out in crystal structures!
There is a shortening of the C-Cl bond length for the complexes participating in halogen bonding, in comparison the the C-Cl bond for the CF3Cl monomer (1.741 Å). Whereas, the C-Cl bond lenghtens for the H-bonding complex. This suggests that there is a change in the s-character of the C-orbital in C-Cl bond .
The energies (Ebind) , at present, can only be interpreted quantitatively. They provide an indication of the interaction strength. They indicate that charged assisted H/halogen-bonding species interact more strongly with CF3Cl. Electrostatics!!!
NBO analysis of C-Cl•••A bonds
| Complex/Species | C-Charge | Cl-Charge | Pol(%C) | s(%C) | s(%Cl) | ENBO (kJ/mol) |
|---|---|---|---|---|---|---|
| CF3Cl•••H3O+ DOI:10042/20394 |
1.195 | 0.015 | 45.07 | 25.42 | 12.95 | 132.88 |
| CF3Cl•••OCH2 DOI:10042/20395 |
1.146 | 0.048 | 48.49 | 28.34 | 13.29 | 2.43 |
| CF3Cl•••Cl- DOI:10042/20398 |
1.106 | 0.121 | 51.23 | 30.71 | 11.78 | 21.34 |
| CF3Cl•••OH- DOI:10042/20392 |
1.080 | 0.149 | 53.62 | 32.15 | 8.90 | 73.43 |
| CF3Cl DOI:10042/20386 |
1.160 | 0.019 | 47.41 | 27.61 | 13.88 | N/A |
Table 7 presents a summary of the key NBO data for the CF3Cl complexes. From the data it can be concluded that the atomic charge of the C-atom in the C-Cl bond increases for H-bonding complexes, while decreasing for halogen bonding complexes (more so for charge assisted species) in comparison to the CF3Cl species (monomer).The inverse is observed for the Cl atomic charge (much greater effect). This indicates that there is a noticable change in the polarisation of the C-Cl bond upon complexation towards the C-atom for halogen bonds and towards the Cl-atom for H-bonds. This can be observed in the Pol(%C) figures which give the percentage polarisation along the C-Cl bond.
There is also a change in the s-character for both the C- and Cl-atoms upon complexation. For H-bonding complexes the s-character of the C-atom decreases, while the s-character of the Cl-atom increases compared to the CF3Cl monomer. The opposite is observed for the halogen bonding species. This observation follows Bent's rule.
Furthermore the ENBO (nA → σCCl*) interaction energy indicates that the greatest electron transfer occurs in the charge assisted complexes.
Molecular orbital analysis of C-Cl•••A bonds
| Complex/Species | LUMO | HOMO | |||
|---|---|---|---|---|---|
| CF3Cl•••H3O+ DOI:10042/20394 |
 |
 |
 HOMO-1 HOMO-1 |
 HOMO-8 HOMO-8 |
 HOMO-14 HOMO-14
|
| CF3Cl•••OCH2 DOI:10042/20395 |
 |
 |
 HOMO-4 HOMO-4 |
 HOMO-5 HOMO-5 |
|
| CF3Cl•••Cl- DOI:10042/20398 |
 |
 |
 HOMO-2 HOMO-2 |
 HOMO-5 HOMO-5 |
 HOMO-11 HOMO-11
|
| CF3Cl•••OH- DOI:10042/20392 |
 |
 |
 HOMO-2 HOMO-2 |
 HOMO-4 HOMO-4 |
 HOMO-5 HOMO-5
|
MOs for CF3Cl•••H3O+
In the CF3Cl•••H3O+ complex (which is H-bonding) the LUMO is predominantly centred on the H3O+ species. The HOMO is predominantly centred on the CF3Cl species, with a p-type orbital on Cl. Furthermore the HOMO is non-bonding with respect to the complex.
A selection of the HOMO-1,HOMO-8 and HOMO-14 are shown in Table 8. The HOMO-1 is σ-antibonding with respect to the complex and is dominated by the p-type orbital on the Cl interacting with the O-atom p-type orbital in H3O+. HOMO-8 represents the bonding interaction between the F-atoms of CF3Cl and the H-atom of H3O+. HOMO-14 is σ-bonding with respect to the complex (not 100% sure of which orbitals).
MOs for CF3Cl•••OCH2
In the CF3Cl•••OCH2 complex (which is Halogen bonding) the LUMO is entirely centred on the OCH2 species. The HOMO is weakly π-antibonding with respect to the complex and is dominated by the HOMO of the formaldehyde species.
The HOMO-4 and HOMO-5 are σ-antibonding and σ-bonding with respect to the complex. HOMO-4 is dominated by the large contribution from CF3Cl whereas HOMO-5 is dominated by the contribution from OCH2.
MOs for CF3Cl•••Cl-
In the CF3Cl•••Cl- complex (Hal-bonding) the LUMO is centred entirely on the CF3Cl species, while the HOMO is the p-LP in the chloride.
HOMO-2 is a weak σ-antibonding interaction between the species in the complex and HOMO-5 and HOMO-11 are both non-bonding.
The MOs of this complex are difficult to interpret as no clear σ-bonding is observed, probably due to poor overlap.
MOs for CF3Cl•••OH-
In the CF3Cl•••OH- complex (Halogen bond) the LUMO is centred entirely on the CF3Cl species. The HOMO is σ-antibonding and is non-linear.
The HOMO-2 and HOMO-4 are the π antibonding-bonding pair between the p-orbitals of CF3Cl and the OH- species, while HOMO-5 is the σ-bonding interaction.
Summary of MOs
Conclusions
Initial studies of a range of CF3Cl complexes partaking in hydogen and halogen bong reveal that Bent's rule can be used to explain the non-bonding phenomena examined. Upon complexation there is a change in the s-character for both the C- and Cl-atoms for both H/Hal-bonds. This leads to an increase/decrease in the elecropositive character of the Cl-atom as confirmed by the NPA charges.