Mod4:Tcg06
Module 1: The basic techniques of molecular mechanics and semi-empirical molecular orbital methods for structural and spectroscopic evaluations
Introduction and Aims
This module aims to rationalise the outcome of known chemical reactions as well as to predict new reactions using two different molecular modeling methods, MM2 and MMFF94 as well MOPAC PM6. These can be used, among many other uses, to minimise the energy of molecules and determine which is the kinetic and which the thermodynamic product of a reaction, but also to model the molecular orbitals of molecules.
Modelling using Molecular Mechanics
The Hydrogenation of a Cyclopentadiene Dimer
Cyclopentadiene readily dimerises via the Diels-Alder reaction. The product of this reaction could be either the endo or exo form depending on whether the reaction proceeds via kinetic or thermodynamic control. It is known that the product of this particular reaction is the endo form; computational techniques will be used to demonstrate whether this is the thermodynamic or kinetic product of the reaction.
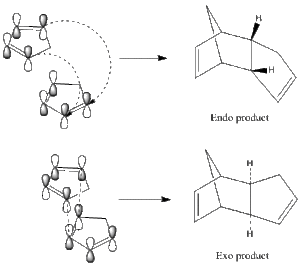
 |
 |
 |
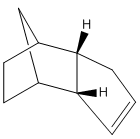 |
Using an MM2,the respective energies of the endo and exo forms were optimised. The exo product was found to have lower energy by 2.12kcal/mol and hence be the thermodynamic product. Since it is known that only the endo product is formed, it can be deduced that the reaction proceeds under kinetic control. This can be explained since the exo- transition state has a more favourable pi-orbital overlap and so there is a lower energy barrier to the reaction than with the endo equivalent. The torsional energy appears to be the largest factor in the energy difference of the molecules. This is a result of the unfavourable steric strain in the endo product between the bridging group and the hydrogens in question compared to the exo product.
| Compound | Stretch kcalmol-1 | Bend kcalmol-1 | Stretch-bend kcalmol-1 | Torsion kcalmol-1 | Non-1,4-VDW kcalmol-1 | 1,4-VDW kcalmol-1 | Dipole/Dipole kcalmol-1 | Total Energy kcalmol-1 |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.29 | 20.58 | -0.84 | 7.65 | -1.42 | 4.23 | 0.38 | 31.88 |
| 2 | 1.25 | 20.85 | -0.84 | 9.51 | -1.54 | 4.32 | 0.45 | 34.00 |
| 3 | 1.25 | 19.16 | -0.84 | 11.08 | -1.64 | 5.80 | 0.16 | 34.96 |
| 4 | 1.10 | 14.52 | -0.55 | 12.50 | -1.07 | 4.51 | 0.14 | 31.15 |
Again using MM2 to optimise the energy, Compound 4 was found to be more thermodynamically stable than Compound 3. The largest difference in energy contributions are from the bending and Van der Waals contributions. This is due to the hydrogenation of the double bond in Compound 3 lowering the strain in the five membered ring, while the increased Van der Waals interactions between the new hydrogens and the bridging group lowers the energy of the molecule further. Due to these factors it is expected that the reaction will proceed under thermodynamic conditions since there is little reason to favour the kinetic reaction pathway.
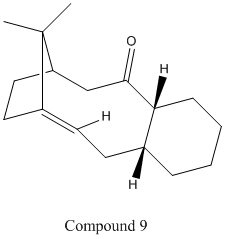
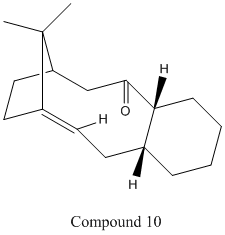
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
The intermediate in the synthesis of Taxol shown exists as two isomers, known as atropisomers, due to the bridging isopropyl group on the main 9-membered ring being adjacent to the alkene group, thus increasing the kinetic barrier to and restricting free rotation within the molecule. In the synthesis, the kinetic product is first produced and this then isomerises to the thermodynamic product. Using both the MM2 and MMFF94 methods, the energy of Compounds 9 and 10 were optimised to find their most stable conformations and to determine which is the most stable of the two.
 |
 |
| Compound | Stretch kcalmol-1 | Bend kcalmol-1 | Stretch-bend kcalmol-1 | Torsion kcalmol-1 | Non-1,4-VDW kcalmol-1 | 1,4-VDW kcalmol-1 | Dipole/Dipole kcalmol-1 | MM2 Total Energy kcalmol-1 | MMFF94 Total Energy kcalmol-1 |
|---|---|---|---|---|---|---|---|---|---|
| 9 | 2.62 | 11.34 | 0.34 | 19.67 | -2.16 | 12.87 | -2.00 | 47.84 | 70.56 |
| 10 | 2.62 | 11.34 | 0.34 | 19.67 | -2.16 | 12.87 | -2.00 | 42.68 | 60.56 |
Although direct comparison between the two methods is meaningless due to different parameters being used by each, both the MM2 and MMFF94 techniques show that the kinetic product for the reaction is Compound 9, while the thermodynamic product is Compound 10. It was found that both molecules had a lower energy when the six-membered ring was put in the chair conformation. The nine-membered ring could also be put into a similarly-shaped conformation to lower the energy in Compound 10, whereas it remained in a twist-boat-like conformation in Compound 9. The higher stability of Compound 10 can be largely explained by the 'hyperstability' of the alkene group next to the bridging group. This is due to the increased strain on hydrogenating the alkene to a single C-C bond as the angle between atoms becomes further from the optimal angle. Although there is a slight decrease in the stretching and bending energy contributions, the torsional and Van der Waals energy contributions increase due to the new, unfavourable small dihedral angles formed by the the new C-H bonds and the bridging group. As such the disfavourability of the hydrogenated taxol intermediate accounts for the very slow reactivity during subsequent functionalisation of the taxol intermediate from Compound 10.
Modelling using Semi-empirical Molecular Orbital Theory
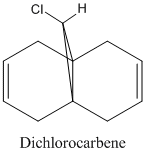
Regioselective Addition of Dichlorocarbene
As well as the classical modelling of molecules, as used in parts 1 and 2, a quantum mechanical method can be used which takes into account the wave-nature of electrons. Using the MM2 and MOPAC/PM6 programs, the molecular orbitals of a diclorocabene compound were modelled.
 |
 |
The HOMO orbital determines the reactivity of Compound 12 and is principally located on the alkene bond syn to the C-Cl bond. This is demonstrates that the electron density is highest across this bond and hence electrophilic attack will take place in this position; chlorocarbenes are highly susceptible to electrophilic addition at this location due to the molecule being electron rich. The HOMO-1 orbital compliments the HOMO orbital, having a high electron density on the alkene bond anti to the C-Cl bond. Compound 12 possesses a plane of symmetry and hence has the point group C1. The large orbital of the LUMO on the alkene bond anti to the C-Cl bond combined with the LUMO+1 orbitals indicate that nucleophilic attack on the chlorocarbene molecule is highly unfavourable.
| Molecular Orbital | Dichlorocarbene |
|---|---|
| HOMO | 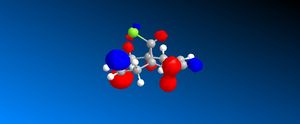 |
| HOMO -1 |  |
| Molecular Orbital | Dichlorocarbene |
|---|---|
| LUMO | 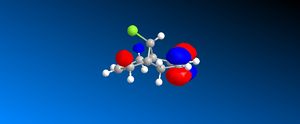 |
| LUMO +1 | 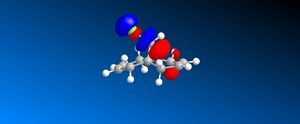 |
| LUMO +2 | 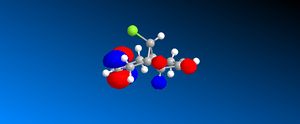 |
Computational techniques can also be employed to predict the spectra of molecules, and these spectra analysed to confirm the molecule's structure. For Compound 12, the IR peak of 689.48cm-1 represents the C-Cl peak, while the IR peaks of 1757.33cm-1 and 1736.98cm-1 represent the alkene bonds syn and anti to the chlorine atom, respectively. The C=C bond has a low polarisability, explaining the weak intensity of these peaks on the IR spectrum.

For Compound 12a, the IR peak of 681.95cm-1 represents the C-Cl peak, while the IR peaks of 1753.76cm-1 represents the alkene bond syn to the chlorine atom. Once again, due to its low polarisability the C=C peak is only of weak intensity on the IR spectrum.
Monosaccharide Chemistry: Glycosidation
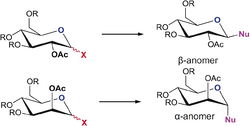
Glycosidation involves replacing the X group at the anomeric centre with a nucleophile, Nu, via an oxonium cation intermediate. The specific anomer produced can be controlled by the position of an adjacent acetyl group which effectively blocks one of the angles of attack of the nucleophile, known as the neighbouring group effect. To form the β-anomer the acetyl neighbouring group must block the bottom face of the molecule, whereas to form the α-anomer the acetyl neighbouring group must block the top face.

To avoid complications the generic R groups with Methyl groups since this does not overload the modelling system. The isomers A', B', C' and D' were all calculated to be higher in energy than their corresponding isomers A, B, C and D respectively since the former four all had increased strain in their structures.
| Compound | Stretch kcalmol-1 | Bend kcalmol-1 | Stretch-bend kcalmol-1 | Torsion kcalmol-1 | Non-1,4-VDW kcalmol-1 | 1,4-VDW kcalmol-1 | Dipole/Dipole kcalmol-1 | MM2: Total Energy kcalmol-1 | PM6: Heat of Formation kcalmol-1 | |
|---|---|---|---|---|---|---|---|---|---|---|
| A | 2.68 | 10.84 | 0.92 | 2.48 | 0.78 | 19.32 | 6.18 | 15.35 | -88.53 | |
| B | 2.86 | 12.41 | 1.03 | 2.29 | 1.45 | 19.15 | 5.96 | 21.31 | -91.66 | |
| C | 2.08 | 13.94 | 0.65 | 8.20 | -3.18 | 18.48 | -0.23 | 31.71 | -88.73 | |
| D | 2.06 | 14.03 | 0.73 | 7.68 | -2.43 | 17.50 | -1.78 | 28.11 | -91.65 |
Structure based Mini Project using DFT-based Molecular orbital methods - The Baeyer-Villiger Reaction
The Baeyer-Villiger is the insertion of an oxygen atom next to the carbonyl group of a ketone to form an ester, using a peroxy-acid. In this case a cyclic ketone is converted into a lactone through two possible reaction pathways to form two products which are isomers of each other.[4] The driving force for the reaction is the very weak and monovalent oxygen-oxygen single bond which cannot carry a positive charge.[5] The proportion of the two isomers produced will depend on the side groups, R, used as well as whether the reaction occurs under kinetic or thermodynamic conditions.
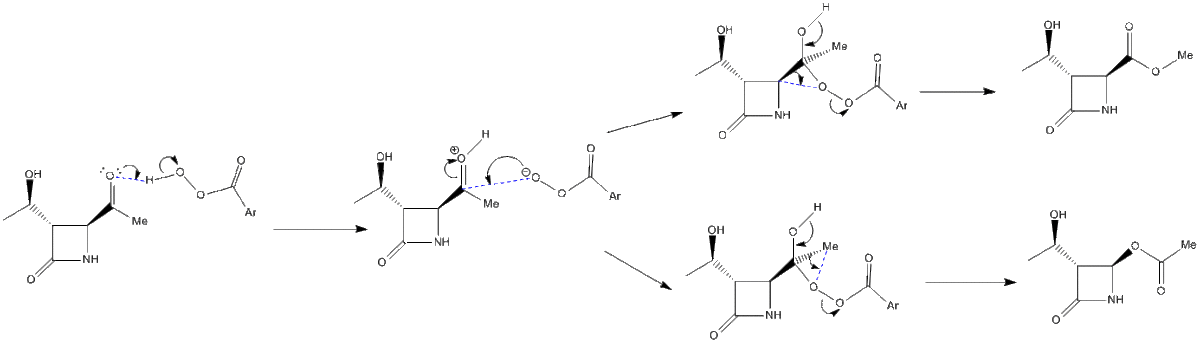
NMR anaylsis was carried out on the two isomers using Gaussian and then compared to literature to determine how accurate the technique was.[7]
Product 1
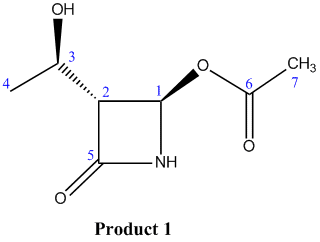
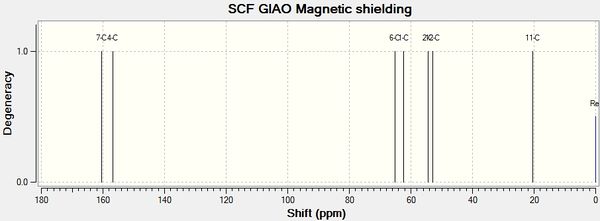
| Exp. Chemical Shift (ppm) | Corrected Exp. Chemical Shift (ppm) | Corresponding Literature Value | Corresponding Carbon Atom |
|---|---|---|---|
| 154.1 | 160.1 | 177.6 | 1 |
| 150.4 | 156.6 | 167.2 | 5 |
| 58.8 | 58.8 | 75.9 | 1 |
| 56.2 | 56.2 | 65.2 | 3 |
| 48.2 | 48.2 | 63.9 | 2 |
| 46.6 | 46.6 | 34.0 | 7 |
| 14.2 | 14.2 | 21.3 | 4 |
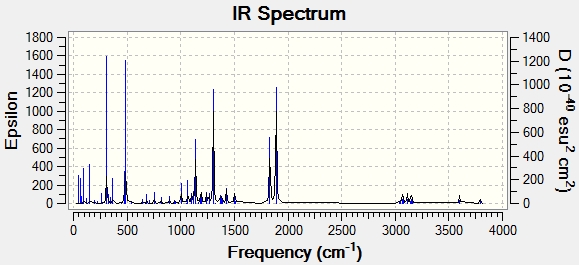
| Frequency cm-1 | Corrected Frequency cm-1 | Corresponding Literature Value | Corresponding Functional Group |
|---|---|---|---|
| 1831.1 | 1684.6 | - | |
| 1889.6 | 1738.4 | 1740 | C=O |
| 3600.3 | 3312.3 | 3330 | N-H |
Product 2
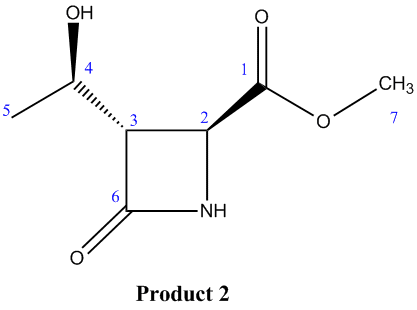

| Exp. Chemical Shift (ppm) | Corrected Exp. Chemical Shift (ppm) | Corresponding Literature Value | Corresponding Carbon Atoms |
|---|---|---|---|
| 166.7 | 172.2 | 170.8 | 1 |
| 158.3 | 164.2 | 168.3 | 6 |
| 77.8 | 77.8 | 69.7 | 2 |
| 66.3 | 66.3 | 64.5 | 4 |
| 64.9 | 64.9 | 64.0 | 3 |
| 23.0 | 23.0 | 50.0 | 7 |
| 21.0 | 21.0 | 21.9 | 5 |
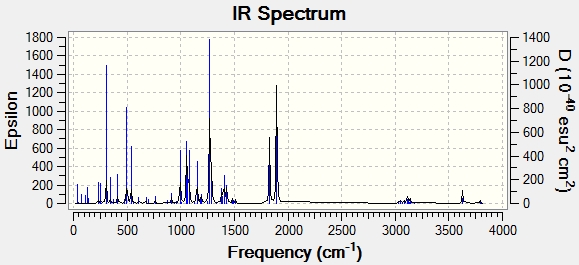
| Frequency cm-1 | Corresponding Literature Value | Corresponding Functional Group | |
|---|---|---|---|
| 1827.3 | 1681.1 | 1721 | C=O |
| 1894.9 | 1743.3 | 1749 | C=O |
| 3627.2 | 3337.0 | 3330 | N-H |
It is evident that both the 13C NMR and IR contain systematic errors in the modelling system and so have been corrected accordingly using 8% lower values in NMR and using the 0.96δcalc + 12.2 for the carbonyl bonds of esters and amides in the IR spectrum. Despite these standard corrections to the modelling results, there is still significant disparity between much of the data obtained experimentally through modelling and from literature sources. This indicates that the Gaussian system is not entirely sufficient to model these particular molecules in a precise way. As such modelling using Gausian could be used as preliminary method of analysing these molecules, which would then need to be backed up by further practical experimentation.
Conclusion
Both classical and quantum mechanical modelling systems are highly useful for modelling molecules and predicting reaction outcomes, however these methods should always be reinforced by practical lab work where possible to ensure the accuracy of the results obtained.
References
- ↑ Clayden, J., Greeves, N., Warren, S. & Wothers, P. Organic Chemistry, 2005, pp916
- ↑ Rezpa,H. Third Year Computational Labs, Module 1:Organic
- ↑ Rezpa,H. Third Year Computational Labs, Module 1:Organic
- ↑ M. Laurent, M. Ceresiat, J. Marchand-Brynaert, J. Org. Chem., 2004,69,p3194-3197
- ↑ Clayden, J., Greeves, N., Warren, S. & Wothers, P. Organic Chemistry, 2005, pp992-996
- ↑ 3rd Year Organic Chemistry, Alan Spivey, Lecture 4 note
- ↑ Lambert,J. et al, 1998, Organic Structural Spectroscopy
