Mod1jsm
Introduction
Computer modelling can be used successfully to model aspects of organic chemistry. The information can be used to rationalise existing reactions as well as predicting the outcome of new reactions. The purpose of the following exercises is to illustrate some examples of molecular mechanics and semi-empirical and DFT molecular orbital theory.
The Hydrogenation of Cyclopentadiene Dimer
Cyclopentadiene dimerisation
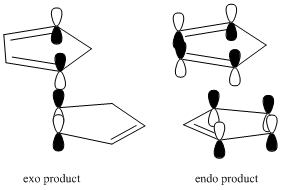
Cyclopentadiene readily reacts in a [4+2] cycloaddition reaction to produce the endo dimer rather than the exo dimer. This observed reactivity can either be thermodynamically controlled or kinetically controlled.

Chem3D was used to define the 2 products and the MM2 force field was used to optimise their geometries. The results gained for the total energy of the products are displayed below:
| Product 2, exo product | Product 1, endo product | |
|---|---|---|
| Energy (kcal/mol) | 31.88 | 34.01 |
As seen from the table, the energy of product 2 (the exo product)is lower in energy than product 2 (the endo product). The difference in energy between the products is 2.13 kcal/mol. From this, it can be seen that the exo product is the thermodynamically more stable product. However the endo product is observed, and so the reaction is under kinetic control.
The diagram above shows the alignment of the molecules needed for the exo or endo products to be produced, and the orbital overlaps. It can be seen that the endo transition state has secondary orbital interactions that do not exist for the exo transition state. This illustrates the kinetic selection of the endo product over the exo product.
Cyclopentadiene dimer hydrogenation
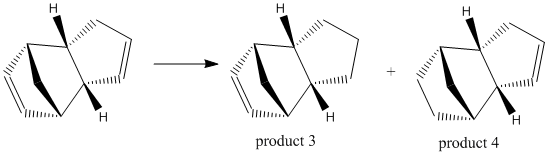
The 2 products of the hydrogenation of the endo dimer product (from above) were defined and their geometries optimised using the MM2 force field:
| Product 3 | Product 4 | |
|---|---|---|
| Stretching | 1.28 | 1.09 |
| Bending | 19.80 | 14.52 |
| Torsion | 10.87 | 12.50 |
| Van de Waals | 5.64 | 4.51 |
| Dipole/dipole | 0.16 | 0.14 |
| Energy (kcal/mol) | 35.70 | 31.15 |
The values in the table relate to the relative contributions of different modes to the energy of the product as a whole. The total energy is lower for product 4 than product 3, with a difference of 4.54 kcal/mol. It can be deduced that product 4 is the thermodynamic product and product 3 is the kinetic product.
The difference in total energy of the molecules is seen to be due to the torsional strain and the bending term. Both of these values are higher in product 3 than product 4, thus leading to a higher total energy. The differences between the molecules for the other terms is negligible compared to the torsional and bending terms.
The double bond next to the bridging CH2 unit is the double bond that is hydrogenated to form product 4, and so is easier to hydrogenate than the other double bond in the molecule. This is supported by the torsional strain being higher in product 3 than 4, which shows that the double bond next to the bridging unit is more strained that the other double bond in the molecule.
Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+ analogue)
Reaction 1
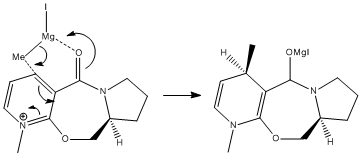
The reaction shown to the right reacts to produce the product with the absolute stereochemistry shown in the scheme.

The reactant in the reaction was defined and the MM2 force field was used to optimise the geometry. Different conformers of the reactant were drawn and minimised using the MM2 force field, with the focus lying on the geometry of the carbonyl group. This gave different minimized geometries with different total energies and dihedral angles. Dihedral angles were measured using the carbonyl carbon and oxygen, along with the adjacent aromatic carbon, and the aromatic carbon adjacent to that one.
The conformers were changed depending on the structure of the 7-membered ring and the 5-membered ring (due to the rigid nature of the carbonyl bond and the aromatic ring, these were left planar). The conformers were then changed by moving the oxygen in the 7-membered ring and the carbon in the 5-membered ring furthest away from the rest of the molecule. These were alternated to sit above and/or below the plane of the aromatic ring. The results are shown below:
| Property | Conformer 1 | Conformer 2 | Conformer 3 | Conformer 4 | Conformer 5 |
|---|---|---|---|---|---|
| 5 membered ring buckle | above plane | above plane | below plane | below plane | flat |
| Ether oxygen atom | above plane | below plane | above plane | below plane | flat |
| Energy of molecule (kcal/mol) | 44.41 | 44.62 | 44.70 | 43.11 | 43.13 |
| Dihedral angle of carbonyl group to aromatic ring | 23.8 | 12.2 | 23.7 | 10.9 | 9.0 |
As seen from the results, the lowest energy conformation (most stable) was the one drawn when both variables were down. The structure is shown below:
Lowest conformation structure |
It can be seen that the optimum dihedral angle produced was 10.9 degrees, but it can also be seen that the carbonyl is always on the top face of the molecule in all minimisations. This is what brings about the selectivity of the methyl group adding to the top face of the molecule. The Grignard reagent co-ordinates to the carbonyl oxygen[2], which sits on the top face of the molecule. When the methyl group is given, it will also add to the top face of the ring. This is shown in the diagram below:
Unfortunately, the MeMgCl component cannot be added when using the ChemBio3D software. If this was possible, the calculations could be refined to include this.
Reaction 2
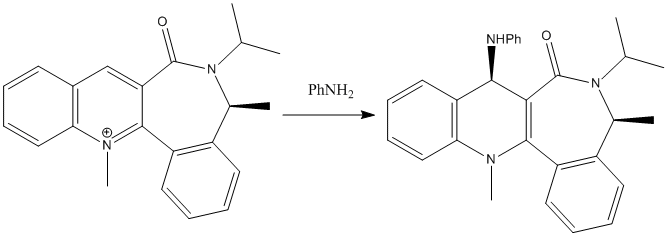
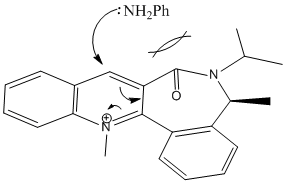
The reaction scheme shows the reaction of the pyridinium ring with aniline. Again, this reaction is stereospecific with regards to where the NHPh group attaches to the pyridinium ring.
In order to find the origin of this control, the reactant in the reaction was defined and the MM2 force field was used to optimise the geometry. Different conformers of the reactant were drawn and minimised using the MM2 force field, with the focus lying on the geometry of the carbonyl group. This gave different minimized geometries with different total energies and dihedral angles. Dihedral angles were measured using the carbonyl carbon and oxygen, along with the adjacent aromatic carbon, and the aromatic carbon adjacent to that one.
The conformers were changed depending on the position of the carbonyl group and the tertiary nitrogen group. Either could sit above or below the plane of the molecule (compared to the Me group that sits above the plane of the molecule). Combinations of these positions were systematically changed and individual minima are shown below:
| Property | Conformer 1 | Conformer 2 | Conformer 3 |
|---|---|---|---|
| Carbonyl group | Above plane | Above plane | Below plane |
| Tertiary nitrogen group | Above plane | Below plane | Below plane |
| Energy (kcal/mol) | 84.17 | 63.74 | 63.55 |
| Dihedral angle | 22.6 | -16.8 | -18.1 |
As seen from the results, the lowest energy conformation (most stable) was the one with a dihedral angle of -18.1. The structure is shown below:
Lowest conformation structure |
Once again, the group added (this time NHPh) adds to the top face of the molecule. It can be concluded that the PhNH2 species will attack on the face opposite to the carbonyl oxygen. This is due to steric repulsion between the groups. The most likely conformer seen for the reactant is where the oxygen lies on the bottom face of the molecule, thus leading the group to add to the top face:
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
Two isomers of an important intermediate in the production of Taxol are shown below:
The isomers are examples of atropisomers. "Atropisomerism is a type of stereoisomerism that may arise in systems where free rotation about a single covalent bond is impeded sufficiently so as to allow different stereoisomers to be isolated"[3]
Isomer A has the carbonyl group point upwards, whereas isomer B has the carbonyl group pointing downwards. Both isomers were defined and their geometries were optimised using the MM2 force field. This process was repeated for the MMFF94 force field. The table below shows the results:
| Molecule | A | B | ||||||
|---|---|---|---|---|---|---|---|---|
| MM2 Energy (kcal/mol) | 55.32 | 49.43 | ||||||
| Torsion (kcal/mol) | 20.17 | 17.51 | ||||||
| MMFF94 Energy (kcal/mol) | 77.60 | 70.66 | ||||||
| Lowest energy conformation |
|
|
As seen from the energy minimisations, molecule B has a lower energy than molecule A, corresponding to when the oxygen is down, rather than up. This is supported for both the MM2 and MMFF94 force fields. Although the actual energy values are different, the energy differences between the molecules for each force field remains similar. It can also be seen that both minimised geometries contain the cyclohexanes in a chair conformation- the lowest energy cyclohexane conformation- supporting that I have found the lowest energy geometries for the molecules.
It is normally rationalised that the strain energy of of an alkene is higher than the alkane derivative, however, hyperstabilised alkenes show the opposite to this. They show negative values to ΔHf(alkane) - ΔHf(alkene).[4]. This is shown in numerous example of olefins at bridgehead positions and medium sized ring positions.[5]. Due to the conformations of the molecules, this situation arises- strain would be increased on hydrogenating the double bond (sp2 to sp3). This renders the double bond more stable than usual.
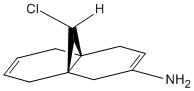
Modelling Using Semi-empirical Molecular Orbital Theory: Regioselective Addition of Dichlorocarbene
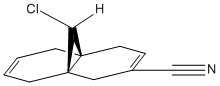
The molecule below (9-ChIoro-1,4,5,8-tetrahydro-4a,8a-methanonaphthalene) was drawn in ChemBio3D:
The molecule was then minimised using the MM2 force field:
Energy of molecule (kcal/mol) = 17.90
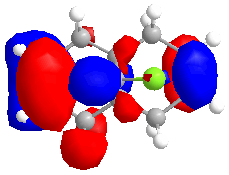
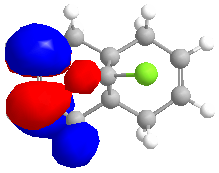
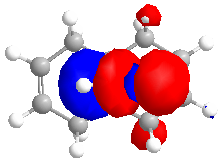
The MOPAC/RM1 method was used to calculate an approximation of the valence-electron molecular wavefunction:
 |
 |
 |
 |
 |
 |
The MO's calculated above can be used to show orbital control of reactivity, namely in the reaction with electrophilic reagents such as dichlorocarbene. Dichlorocarbene readily attacks alkene double bonds in a cycloaddition reaction.
It can be seen from the MO diagrams that there is discrimination between the alkene double bonds that are present in the molecule. The HOMO shows that more electron density sits on the alkene double bond that is endo to the chlorine atom in the molecule. The double bond has more electron density that the other double bond, and so will be more susceptible to electrophilic attack.
The distances between the exo and endo double bond carbons and the central bridgehead carbon was measured on my minimised structure:
| Exo carbon to central bridgehead carbon | Endo carbon to central bridgehead carbon | |
|---|---|---|
| Distance (Angstrom) | 2.98 | 3.22 |
This shows that the molecule is distorted so that the exo double bond is bent towards the central bridgehead carbon more than the endo double bond. This can be explained by an antiperiplanar relationship between the Cl-C σ* orbital and the exo π orbital(HOMO-1). This interaction would stabilise the exo double bond and thus cause the bond to be less susceptible to electrophilic attack, agreeing with the previous results.[6]
The product from the hydrogenated of the exo double bond was defined:
The geometry of the molecule was minimised using the MM2 force field:
Energy of molecule (kcal/mol) = 24.82 kcal/mol
Both molecules were then subjected to a Gaussian calculation in order to calculate the vibrational stretching frequencies and IR spectra:
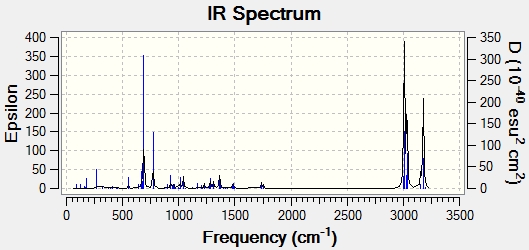 |
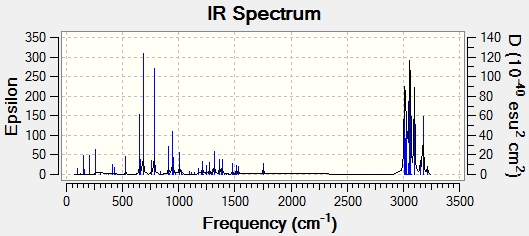 |
| 9-ChIoro-1,4,5,8-tetrahydro-4a,8a-methanonaphthalene | Hydrogenated product | |
|---|---|---|
| C=C bond stretch frequency (cm-1) | 1757.4 | 1753.7 |
| C=C bond stretch frequency (cm-1) | 1737.1 | - |
| C-Cl bond stretch frequency (cm-1) | 770.9 | 780.4 |
As seen from the stretching frequencies, the C-Cl stretch occurs at slightly different frequencies for each molecule. The diene has a lower C-Cl stretch, suggesting that the bond is weaker. This can be rationalised from the previous data. In the diene, the exo double bond is present,and so as mentioned before, there is overlap between the C-Cl σ* and π orbitals. This overlap will weaken the bond due to the antibonding nature of the σ bond that is overlapping. This causes the decrease in bond strength and thus a decrease in stretching frequency. This overlap is not seen for the hydrogenated product due to the absence of the exo double bond, and so the C-Cl bond is stronger and so is seen at a higher frequency.
The other difference between the two is the absence of one of the C=C stretches in the hydrogenated product. Both of the molecules have a C=C stretch at around 1757cm-1 which is for the endo double bond, present in both molecules. The additional stretch in the unhydrogenated product is for the exo double bond. The frequency of this stretch is lower, and so the bond strength is weaker (lower bond energy). This agrees with the above results that the exo double bond has less electron density (thus weaker). This result supports the previous results.
To test the results above even more, I have modified the structure of the original molecule. Both alkene bonds are present, but a hydrogen has been swapped for a CN group on the exo double bond. CN is a strongly electron withdrawing group and so the electron density should decrease, decreasing the bond strength and thus the stretching frequency.
| Bond | C-Cl stretch | C=Cl endo stretch | C=Cl exo stretch |
|---|---|---|---|
| Stretching frequency | 765.7 | 1756.6 | 1706.5 |
The results above agree with this. The vibration for the other double bond remains unchanged from previous results, following that no modification has been made to this alkene.
The C-Cl stretching frequency has decreased compared to both the unhydrogenated and hydrogenated molecules previously calculated. Thus suggests that the bond is weaker, and so overlap is more strong with the exo double bond. This can be explained by the orbital that it is overlapping with will pull the electron density more strongly due to the CN group, thus depleting electron density in the C-Cl bond more than the hydrogen-bearing exo double bond.
To compliment this, I have drawn a structure with an electron donating group attached to the exo alkene:
The geometry was minimized with the MM2 force field and the vibration frequencies were calculated:
| Bond | C-Cl stretch | C=Cl endo stretch | C=Cl exo stretch |
|---|---|---|---|
| Stretching frequency | 765.1 | 1755.6 | 1745.0 |
Once again, the results fit with the prediction that the endo double bond should remain unchanged, whereas the exo double bond should occur at a higher frequency due to higher electron density thus creating a stronger bond.
The C-Cl stretching frequency is again lower than the first two molecules containing just hydrogen or no exo double bond. This suggests the overlap is larger and the bond weaker. This can be due to the fact that there is more electron density now populating the anti-bonding C-Cl orbital, thus weakening the bond and causing the stretching frequency to decrease.
It can be seen that electron withdrawing and electron donating groups both have the expected result on C=C bond strength and stretching frequency, but they both also decrease the strength of the C-Cl bond, and cause the stretch to appear at a lower frequency in IR spectra.
Structure based Mini project using DFT-based Molecular orbital methods
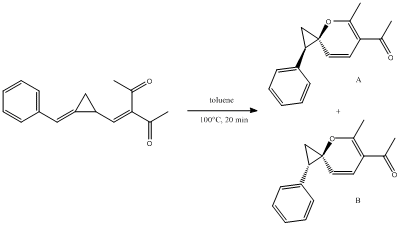
The reaction I have chosen to study is the "thermally induced electrocyclic reactions of methylenecyclopropane (MCP) methylene diketone derivatives into spiro[2.5]octa-3,5-dienes".[7]
(E)-3-((2-benzylidenecyclopropyl)-methylene)pentane-2,4-dione was heated in toluene at 100°C and a product, trans-spiro[2.5]octa-3,5-diene, was obtained in 56% yield. This differed from the expected result of a cyclopentene product derived from a Cope rearrangement.
The cis isomer of the product, cis-spiro[2.5]octa-3,5-diene, could not be isolated due to its instability.
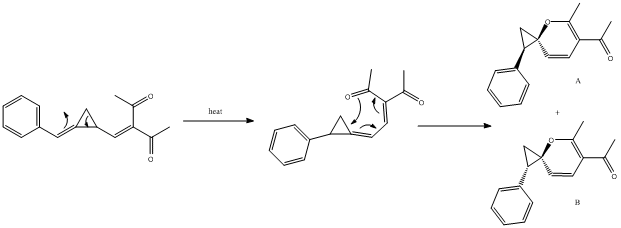
A plausible reaction mechanism is outlined below:[7]
Energy minimisation
The MM2 force field was used to minimise the energy of the isomers in ChemBio3D. However, there is an element of conformational change in the molecules and so each isomer was minimised twice from different starting positions to ensure that I had acheived the real minimum geometry.
The molecules are fairly rigid and have well defined stereochemistry. However, the ketone group in the molecule can be rotated around into different positions.
Product A yielded two different minimum geometries:
Total Energy: 28.44 kcal/mol
Total Energy: 25.31 kcal/mol
Product B also yielded two different minimum geometries:
Total Energy: 28.12 kcal/mol
Total Energy: 24.50 kcal/mol
Both conformers are lower in energy for product B than product A. The higher energy minimum is 0.3131 kcal/mol more stable for product B, and the lower energy minimum is 0.8092 kcal/mol more stable for product B also. This result indicates that the reaction is under thermodynamic control and product B is the lower energy and more stable product that is seen as the product.
Free energy calculation and differences
From the vibrational calculation, the free energy is found for each product:
Product B
Sum of electronic and thermal Free Energies= -769.519801
Product A
Sum of electronic and thermal Free Energies= -769.519490
The difference in free energies:
-769.519801 - (-769.51949) = 0.000311 Hartree
Energy difference= 0.21 kcal/mol
Product B is 0.21 kcal/mol more stable than product A
The result for this calculation supports the result from the experimental data. Product B is expected to be seen, and product A is expected to be present in trace amounts or not easy to isolate. From this result, it can be supported that the reaction is under thermodynamic control.
IR spectral calculation and assignment
The IR spectra of products A and B were calculated and the peaks were matched to peaks from the literature.
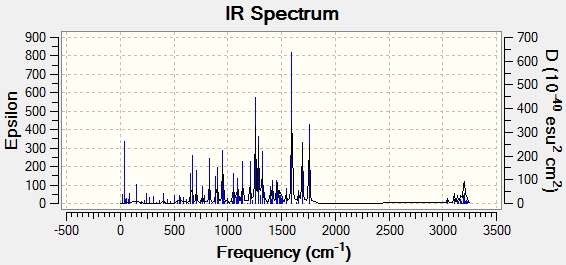 |
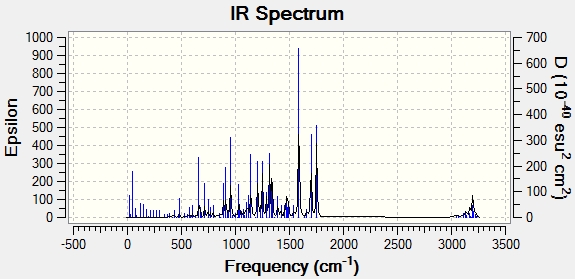 |
| Peak assignment | Literature values for product A(cm-1)[8] | Computed values for product B (cm-1) | Computed values for product A (cm-1) |
|---|---|---|---|
| CH3 stretch | 3062 | 3061 | 3048 |
| CH3 stretch | 3029 | 3044 | 3045 |
| - | 2946 | - | - |
| - | 2925 | - | - |
| - | 2855 | - | - |
| C=O | 1718 | 1752 | 1757 |
| C=C | 1603 | 1699, 1587 | 1693, 1596 |
| CH3 | 1496 | 1495 | 1495, 1494 |
| C-H | 1453 | 1456 | 1457 |
| CH3 | 1424 | 1421 | 1418 |
| Aromatic | 1363 | 1361 | 1366 |
By comparing the IR peak sets for products A and B, it can be seen that the major peaks match up well. The vibrations that peak frequencies appear at (seen from Gaussview calculation) match very well with literature values for each bond frequency range. This supports that the product obtained in the article, is one of the products A or B. Product B fits slightly better with the data, however, big differences in the spectra between the 2 products is not expected- both products have the same number and type of bonds. IR spectoscopy is not a good method for distinguishing between isomers, but it can be used as evidence that the right molecule has been made.
NMR analysis
Below are the NMR spectra that were calculated for each isomer:
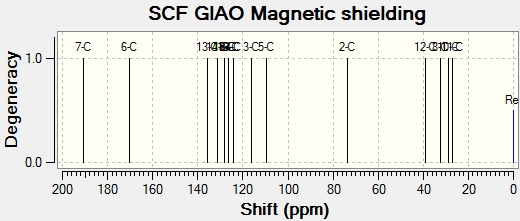 |
 |
This is the structure of the product B:
Lowest conformation structure |
This is the structure of the product A:
Lowest conformation structure |
| Atom number | Literature shift (ppm)[8] | Product A shift (ppm) DOI:10042/to-5708 | Product B shift (ppm) DOI:10042/to-5707 | Product A shift difference (ppm) | Product B shift difference (ppm) |
|---|---|---|---|---|---|
| 7 | 196.1 | 191.7 | 190.8 | -4.4 | -5.3 |
| 6 | 166.7 | 171.6 | 170.2 | 4.9 | 3.5 |
| 13 | 135.9 | 135.5 | 135.8 | -0.4 | -0.1 |
| 14 | 128.5 | 130.2 | 131.1 | 1.7 | 2.6 |
| 15 | 128.3 | 127.8 | 127.9 | -0.5 | -0.4 |
| 17 | 128.3 | 127.2 | 128.3 | -1.1 | 0 |
| 4 | 126.4 | 126.2 | 124.0 | -0.2 | -2.4 |
| 16 | 126.4 | 125.1 | 126.4 | -1.3 | 0 |
| 18 | 123.4 | 124.3 | 128.1 | 0.9 | 4.7 |
| 3 | 116.1 | 119.0 | 116.1 | 2.9 | 0 |
| 5 | 113.8 | 115.6 | 109.5 | 1.8 | -4.3 |
| 2 | 65.2 | 69.5 | 73.8 | 4.3 | 8.6 |
| 12 | 31.5 | 40.2 | 38.9 | 8.7 | 7.4 |
| 8 | 29.3 | 33.3 | 32.2 | 4.0 | 2.9 |
| 11 | 20.5 | 24.6 | 26.9 | 4.1 | 6.4 |
| 10 | 19.7 | 24.2 | 28.9 | 4.5 | 9.2 |
As seen from the data, there is not much variation in the NMR between product A and B. It can be seen that product A fits better for certain carbons, whereas product B fits better than product A for other carbons. Here, the computations are not accurate enough to be able to distinguish between the isomers effectively- a more accurate and precise method is needed to accurately use this method to distinguish between the products.
The3J coupling values for the 1H NMR have been calculated for the isomers and compared to the literature values:
3J coupling values (Hz)
Literature [8]
10.4
7.6
9.6
Product A
10.27
9.93
8.22
Product B
10.33
9.98
8.22
Again, both isomers appear to have very similar results, and there is nothing to point towards product B over product A.
Discussion
It is clear from the results, that product B is the most likely product to be produced in the reaction due to the lower energy compared to product A. Both IR and NMR calculations support the production of one of the products, but the computations have failed to differentiate between the products. The calculations are clearly not accurate enough to be able to say that product B has been produced over product A- the NMR changes must be smaller than the margin of error on the calculations I used.
In the journal, it is discussed briefly that the product from a Cope rearrangement is not seen (although expected). There is no clear evidence in the report to back up this finding. As a contrast, it would be a good idea to calculate the IR and 13C NMR spectra for the product(s) formed from this reaction. It would be expected that the IR spectrum would match fairly well with the literature spectrum because the products would vary only slightly from the reported products, and would contain the same types of bonds. The real sign to whether the authors were correct, would be with the NMR fitting. It would be clear whether the product(s) that were ruled out fit better to the literature data. Again, the NMR spectra for the ruled out product(s) would be similar to the NMR for the reported products, and so a wrong assignment would not be too hard to achieve. If more time was available for the project, I would carry out this research.
An 1H NMR spectrum for product A and product B may be a better choice for differentiating between the products, however, it was not possible for me to compute one due to the complexity of the spectra and the time aspect of this report. Another good technique to use would be x-ray diffraction. The product was an oil when it was produced, and so the product would need to be crystallised (if possible) for this measurement to be taken.
Conclusion
Computational methods can be used rather effectively to predict the outcomes of reactions, as well as rationalising the outcomes of reactions when they are known. It is possible to predict with good accuracy the spectra that can be measured for molecules and products. However, there is a limit to how far the calculations can go before they start to break down- as shown in the mini-project. Predicted spectra match well for the products, but more complicated techniques, or perhaps more time for more measurements are need to be used to effectively differentiate between some isomers. This is where the technique begins to break down at the level I am using it.
References
- ↑ Peptide antibiotics: Steric Effects vs Secondary Orbital Overlap in Diels-Alder Reactions DOI:10.1021/jo00384a016
- ↑ Regio- and Stereoselective Control in the Addition of Grignard Reagents to the Pyridine Ring System DOI:10.1021/jo00356a016
- ↑ Atropisomerism, biphenyls and the Suzuki coupling: peptide antibiotics: DOI:10.1039/b001971m
- ↑ Cross-coupling of a functionalized highly pyramidalized alkene DOI:10.1016/S0040-4020(01)00802-X
- ↑ Hyperstable olefins: further calculational explorations and predictions DOI:10.1021/ja00274a016
- ↑ A Molecular Orbital and Crystallographic Study of the Structure and π-Facial Regioselectivity of 9-ChIoro-1,4,5,8-tetrahydro-4a,8a-methanonaphthalene: DOI:10.1039/P29920000447
- ↑ 7.0 7.1 Thermally Induced Electrocyclic Reaction of Methylenecyclopropane Methylene Diketone Derivatives: A Facile Method for the Synthesis of Spiro[2.5]octa-3,5-dienes: DOI:10.1021/ol102002f
- ↑ 8.0 8.1 8.2 Thermally Induced Electrocyclic Reaction of Methylenecyclopropane Methylene Diketone Derivatives: A Facile Method for the Synthesis of Spiro[2.5]octa-3,5-dienes- Supporting Information: DOI:10.1021/ol102002f