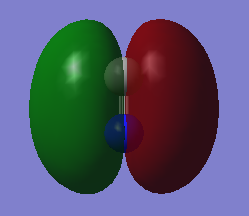Mod1485
My Molecules
NH3
NH3 optimal
Data:
calc Method: RB3LYP
Basis Set: 6-31G(d.p)
Energy: -56.55776873 au
RSM Grad: 0.00000485 au
Point Group: C3V
Bond Angle: 105.741o
Bond Length: 1.01798 au
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Predicted change in Energy=-5.986273D-10
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.018 -DE/DX = 0.0 !
! R2 R(1,3) 1.018 -DE/DX = 0.0 !
! R3 R(1,4) 1.018 -DE/DX = 0.0 !
! A1 A(2,1,3) 105.7412 -DE/DX = 0.0 !
! A2 A(2,1,4) 105.7412 -DE/DX = 0.0 !
! A3 A(3,1,4) 105.7412 -DE/DX = 0.0 !
! D1 D(2,1,4,3) -111.8571 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
NH3 |
Media:HenryW-NH3_OPTIMISED.LOG
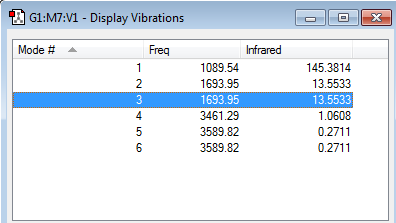
Questions - Vibration
No. Nodes: N=4 3*4 - 6 = 6
Degenerate Nodes: Nodes 2 and 3 are degenerate as well as 5 and 6. This is due to them having the same frequency.
Bond Bending: 1,2,3
Bond Stretching: 4,5,6
Symmetry: 4 shows a very high level of symmetry during vibration.
Vibration 1 is the Umbrella vibration.
I would expect 4 unique bands in a spectrum of gaseous Ammonia due to there being 6 vibrations but there are 2 degenerate states.
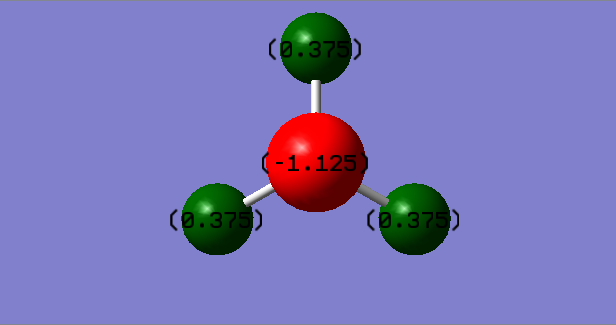
Charges
N= -1.125 eV
H= +0.375 eV
This is close to my predicted charge distribution as the difference in electronegativity is 0.9. This is under 1.7 so therefore covalent but with slight ionic character. This means I would expect a higher negative charge distribution on the N atom as it draws the electron density towards it.
H2
H2 optimal
Data:
calc Method: RB3LYP
Basis Set: 6-31G(d.p)
Energy: -1.17853936 au
RSM Grad: 0.00000017 au
Point Group: D*H
Bond Angle: 180o
Bond Length: 0.74279 au
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
Predicted change in Energy=-1.164080D-13
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 0.7428 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
H2 |
No negative therefore optimisation is okay
Charges are equal as a simple diatomic molecule of the same atom type.
N2
N2 optimal
Data:
calc Method: RB3LYP
Basis Set: 6-31G(d.p)
Energy: -109.52412868 au
RSM Grad: 0.0000006 au
Point Group: D*H
Bond Angle: 180o
Bond Length: 1.1055 au
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
Predicted change in Energy=-3.401156D-13
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.1055 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
N2 |
Media:HENRYW-N2_OPTIMIMISE.LOG
No negative value so optimisation is okay.
Haber-Bosch energy
E NH3 = -56.55776873 au
E NH3 * 2 = -113.11553746 au
E N2 = -109.52412868 au
E H2 = -1.17853936 au
E H2 * 3 = -3.535618 au
ΔE=2*E(NH3)-[E(N2)+3*E(H2)] = -0.05579078 au
= -146.47869289Kj/mol
The literature value for this is -91.4Kj/mol (45.7*2) 1. The calculated value varies rather significantly from the literature value, however the value is correct to be a negative as the process is exothermic. The difference in the values is an unknown error.
As N2 is the large negative value than H2 it means it is the more stable of the two reactants. This is due to the energy needed to break it apart is the positive value of the net energy, meaning the N2 is 108.34558932 au units more stable than H2. This is expected as the N-N triple bond is seen to be the second strongest bond, behind only C=O.
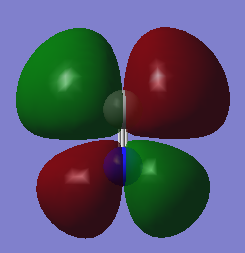
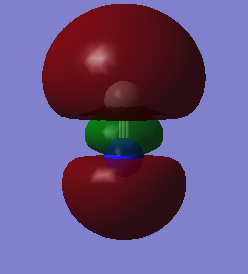
MOs N2
This is the MO formed by the combination of the N's 2s and the p orbitals. Occupied. Energy = -1.12383
This is the MO formed by the combination of the N's 2s orbitals with the p, however this is anti-bonding. Occupied. Energy = -0.55342
This is the MO formed by the combination of the N's SP hybrid orbitals to form a bonding orbital. This is the HOMO orbital. Occupied. Energy = -0.42688
This is the anti-bonding orbital, for the pi bonds, formed by the N's SP hybrid orbitals. This is the LUMO orbital. Unoccupied, hence N2's high negative energy as no anti-bonding orbitals are filled. Energy = 0.02412
The reason N2 is such a strong bond is the Mos ocupied in the p section are all bonding. None of the anti-bonding orbitals are filled. 2
My Choice
CN-
calc Method: RB3LYP
Basis Set: 6-31G(d.p)
Energy: -92.82453153 au
RSM Grad: 0.00000704 au
Point Group: C*V
Bond Angle:180 o
Bond Length: 1.18409 au
Item Value Threshold Converged?
Maximum Force 0.000012 0.000450 YES
RMS Force 0.000012 0.000300 YES
Maximum Displacement 0.000005 0.001800 YES
RMS Displacement 0.000008 0.001200 YES
Predicted change in Energy=-6.650383D-11
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.1841 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
CN- |
Media:HENRYW-CN-_OPTIMISED.LOG
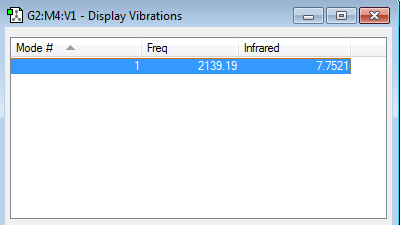
This is a stretching action. As CN- is di-molecular only 1 rotation is found
Charges
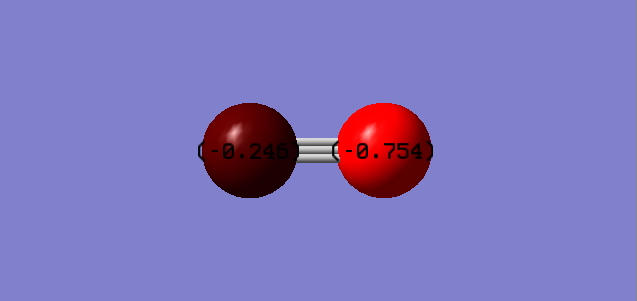
C = -0.246 eV
N = -0.754 eV
This shows the majority of the 1- charge the CN ion has over the N, assumingly due to it's higher electronegativity, however in theory the negative charge rests upon the Carbon atom, as this is the atom that acts as the nucleophile in mechanisms. The unoccupied sigma orbital of the Carbon holds the negative charge.
Orbitals


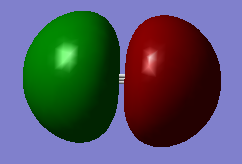
This is the CN- SP1 orbital. As shown in the second photo the smaller lobe is clearly seen and shows the classic hybridized shape. Occupied. Energy:-0.56195 au
This is the anti-bonding orbital for the 2s. Occupied. The distortion of the orbital is due to the difference in electronegativities of the two atoms. Energy:-0.10626 au
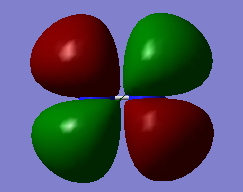
As CN- is SP1 hybridized it has two pi orbitals surrounding the one sigma bond to create a triple bond. This is one of the two degenerate pi bonding orbitals and is the same as that found in the N2 Mos. Occupied. Energy:-01696 au
This is the anti-bonding orbital for the pi system. This is the LUMO so is therefore unoccupied. Energy:0.35435 au
This is the sigma bond withing the cyanide ion. Bonding orbital. This is the HOMO so occupied. This is similar to the HOMO of the N2 Homo although due to the charge placed upon the C atom the e- density is shifted across to it, thus creating a larger lobe around the C atom. Energy:0.01857 au
This is the unoccupied anti-bonding sigma orbital. Energy:0.59206 au
Conversion rates
Au values:
Energy = Hartree (*2625.5 to Kj/mol)
Length = Bohr (*5.2918e-11 to m)
references
1. Haber process for Ammonia synthesis. Jayant M Modak. August 2002
2. http://www.huntresearchgroup.org.uk/teaching/teaching_comp_lab_year1/10_mos_n2.html (09/03/17)
3. http://chemistry.stackexchange.com/questions/18449/cyanide-ion-non-bonding-lone-pair (10/03/17)