Mod.MAP107
Module 1:
Section 1A: Cylo Pentadiene dimers
The first section of this investigation will look into the thermo dynamic stabilities of the cyclopentadiene dimer’s and the resulting products after they have undergone a hydrogenation reaction.
Molecules to be modelled:

These molecules shall be modelled using ChemBio3D Ultra. The Molecular Mechanics (MM2) method shall be implemented in order to calculate the energies of these molecules. From this conclusions shall be drawn on the reaction mechanisms, and the contributions of different stretches and forces that act on the hydrogenated products.
Cyclopentadiene undergoes a Diels Alder reaction with its self to form a dimer. This reaction is slow at room temperature however, energetically favourable.
When considering a Diels Alder reaction, stereo selectivity has to be taken into account. Two products are generated in such a reaction; the endo and exo products. These “labels” refer to the transition state by which the product was formed.
These products result from the endo or exo attack of the dienophile on the diene.
These attacks are shown diagrammatically below:
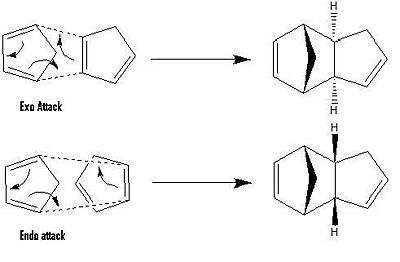
Endo vs Exo attack:
The Endo rule:
The endo rule of stability states that the endo attack normally leads to a more stable transition state. The major product of the reaction will hence be the endo product, even if the endo product is thermo dynamically unstable relative to the exo product.
Modelling of the endo and exo products will reveal if the endo rule holds true and weather the reaction is driven by kinetic or thermo dynamic control.
Modelling and Results of the Endo and Exo products:
Diagramatic models:
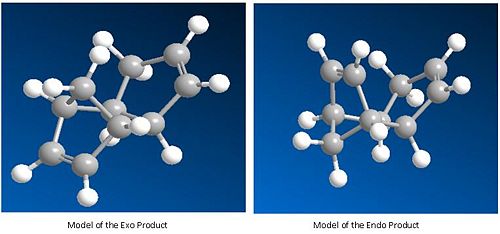
Exo Product (1):
Endo Product (2):
Endo Product:
Isomer 1 |
Results table:
| Energy of Exo Product/Kcal/mol | Energy of Endo Product/Kcal/mol |
|---|---|
| 31.90 | 34.90 |
Analaysis of Results
From the results it can be seen that the endo product has a higher energy than the Exo Product.from this it can be concluded that the endo rule holds true in this reaction and the kinetic reaction happens in order to produce the Thermo dynamically unfavourable product.
Modelling of the hydrogenated endo Products
Diagramatic models:
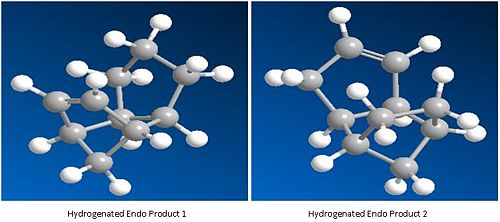
Molecule 3:
Molecule 4:
Results table: Total Energy of Molecules
| Energy of Endo Dimer/Kcal/mol | Energy of hydrogenated endo product 1/Kcal/mol | Energy of Hydrogenated product 2/Kcal/mol |
|---|---|---|
| 34.01 | 35.93 | 31.17 |
Results tables: break down of energies
| Bend Energy/Kcal/mol | Stretch Energy/Kcal/mol | Torsion Energy/Kcal/mol | VDW Energy/Kcal/mol |
|---|---|---|---|
| 20.83 | 1.26 | 9.51 | 4.31 |
| Bend Energy/Kcal/mol | Stretch Energy/Kcal/mol | Torsion Energy/Kcal/mol | VDW Energy/Kcal/mol |
|---|---|---|---|
| 18.96 | 1.23 | 12.1 | 5.74 |
| Bend Energy/Kcal/mol | Stretch Energy/Kcal/mol | Torsion Energy/Kcal/mol | VDW Energy/Kcal/mol |
|---|---|---|---|
| 14.54 | 1.097 | 12.5 | 4.5 |
Analysis of Results:
As we can see from the results, one hydrogenation leads to a more stable thermodynamic compound (Hydrogenation product 2 (4)) and one leads to an increase in the energy of the molecule (hydrogenation product 1 (3)). From this it can be concluded the preferred product is (4). A break down of the Energy components will reveal more information to why this may be.
Product 3 has a much lower bend energy than product 4. This can be explained through analysisng the bond strain of the two double bonds. This can again be achieved through using ChemBio3D. Please the the picture below for reference.

The optimum geometry for a carbon-carbon double bond is 120 degrees. It can be seen from the picture that niether of these two double bonds has this geometry. However one five membered ring is much more strained than the other, with bond anles of 108 degrees in comparison to 112 and 113. A hydrogenation of this double bond would relieve the bond strain more than a hydrogenation of the other double bond. This in turn would reduce the energy more. Hence It can be concluded that the second hydrogenation product (4) is the preferred product and this is due to greater relief in bond strain.
From these results it would be expected that the hydrogenation reaction that leads to the formation of prodyct 4 has a positive Olifin Strain Energy (OSE) as the reaction that forms 3 may have a negative OSE. This will be discussed more in the Taxol intermediate section. However it may be possible to describe one of the double bonds as being hyper stable.
A further explanation for the increase in energy between the endo diemer and the first hydrogenation product(3) could be the increase in van der waals forces between hydrogens.
References
http://en.wikipedia.org/wiki/Diels%E2%80%93Alder_reaction Diels-Alder reaction
Section 1B: Nucleophillic Attack of Pyridinium rings
In this section two nucleophillic attacks of a pyridinium NAD+ analogue shall be modelled. The first reaction involves the attcak of methyl magnesium iodide (a Grignard reagent) on Prolinol, and the second reaction involves the attak of Aniline on a a molecule containing a pyridinium NAD+ analogue.
The two reactions are very sterio selective. Through molecular modelling the reason for this selectivity should be determined. Again ChemBio3D can used in modelling this. The reactant molecules will be modelled in order to deterine there lowest energy geometries, and then through the analysis of dihedral angles, the properties of the nuclephiles shall be examined in order to see how they will interact with the carbonyl group.
Molecules and Reactions to be Modelled
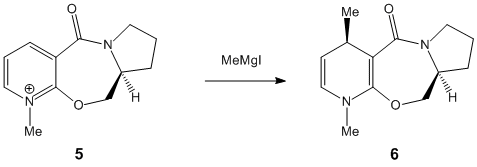
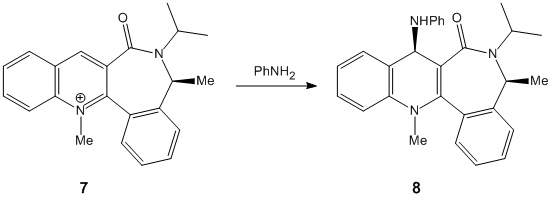
Molecules 5 and 7 are to be modeled.
Diagramatic Models and Optimum Geometry Process:
The pictures below show the lowest energy geometries generated in the modelling process.

Molecule 5 had a total energy of 43.107Kj/mol. Molecule 7 had a total energy of 62.67Kj/mol.
Process involved in modelling molecule 5:
The picture below shows how the lowest energy geometry was achieved through moving the carbonyl group. observe how the oxygen, high lighted in yellow moves from its starting position to a new location after the MM2 calculation is run. Not all the steps are shown, however the process was repeated with other geometries.

Process involved in modelling molecule 6:
The picture below shows how the lowest energy geometry was achieved through moving the carbonyl group. observe how the oxygen, high lighted in yellow moves from its starting position to a new location after the MM2 calculation is run. Not all the steps are shown, however the process was repeated with other geometries.

Analysis of Modelling
When modelling a molecule it is important to realise that molecules are not stiff and rigid, and that 100's of different conformations and geometries may exist. When applying an MM2 calculation to a molecule, the energy value generated is one of many different possible answers. This is a real life problem inherent of any molecule. ways to over come and improve this may be to generate all the different energy conformations and take an average, or take a sample of commonly obtained energies and take an average. However these methods would take a long time and can't be implemented in this section. More advanced computer programmes such as gaussen exist to solve these problems However these are discussed and implemented in module 3 and are beyond the scope of this exercise.
Modelling Nucleophillic Attack on Molecule 5:
The nucleophillic attack being modelled involves a Grignard reagent. Grignard reagents have both an electrophillic part (in this case the magnesium) and a nucleophillic part (in this case the alkyl group). Selectivity of this reaction will be governed by the Magnesium's ability to co-ordinate to the carbonyl group of molecule 5.
The nucleophillic attack of this reaction has been shown to be very selective. With the alkyl group being delivered to the same face of the molecule as the ether (this shall be referred to as the top face with reference to the picture below). The selectivity can be explained by looking at the geometry of the carbonyl group relative to the pyridinium ring, and specifically, the site of nucleophillic attack.
As the pridinium ring is aromatic and fulfills Huckels rules an is planar. If we measure the dihedral angle between the ring and the carbonyl group we find that the carbonyl group is not in the same plane as the pyridinum ring and is on the top face (with reference to the picture below) the Dihedral angle has thus been measured to be 10 degrees.

Molecule 5:
From this we can now model the reaction and give reference to the sterio chemistry. As the electrophillic magnesium will co-ordinate to the carbonyl, it too, will be on the top face of the molecule and will hence deliver the methyl group to the top face of the pyridinium ring.
This is illustrated below with the full reaction scheme shown below;
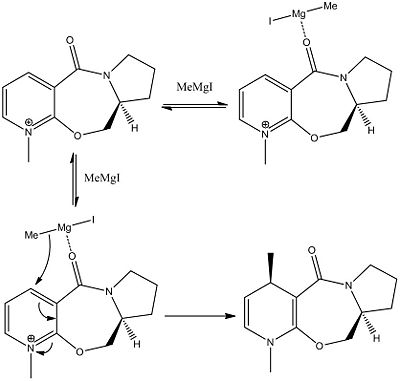
Modelling Nucleophillic Attack on Molecule 7:
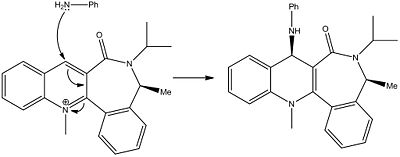
This nucleophillic attack models the attack on aniline. Again this reaction is very sterio selective. Analine is a large aromatic nucleophille. This means that there is a large amount of electron density above and below the phenyl ring. The carbonyl group also possess a large amount amount of electron density on the carbon oxygen double bond and on the oxygen. When aniline attacks the pyridinium ring it will avoid the carbonyl in order to reduce the the repulsive culombic forces.
The faces of the molecules are defined in the picture below. The dihedral angle between the carbonyl and the pyridinium ring is also shown below, and has been calculated as -20 degrees.

Molecule 7:
It can be seen from the picture shown above, that the carbonyl is in the bottom face of the molecule. This means that the aniline will attack the opposite face, which in this case is the top face. This explains the sterio selecticity.
The reaction scheme for this reaction is shown below:
Problems and Improvements to Experiment
References
Grignard Reagents http://pubs.acs.org/doi/abs/10.1021/ba-1959-0023.ch008?prevSearch=grignard&searchHistoryKey=
Selction 1C: Modelling of Taxol Intermediates
In this section an intermediate in in the synthesis of the anti cancer drug taxol shall be modelled. Again ChemBio3D shall be used along with the molecular mechanics calculation MM2 to calculate the lowest energy isomer.
The isomers being modelled are antropisomerism (see atropisomerism). This means there is limited rotation about a single bond within the molecule. In this intermediate there is limited rotation between the cyclo hexane ring and the carbonyl group. This is shown in the picture below, which shows the carbonyl on the top and bottom faces of the molecule. Upon initial synthesis of this molecule, both isomers will be formed. After a period of time only the thermodynamically more stable isomer will remain as the rotation around the single bonds takes place. through the Use of sterio electronics the relative stabilities of the molecules and carbon carbon double bond shall be explained.
Molecules to be Modelled
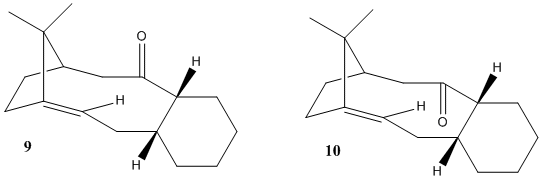
Diagramatic Models and Optimum Geometries
The models below show the two isomers in there lowest energy geometries.
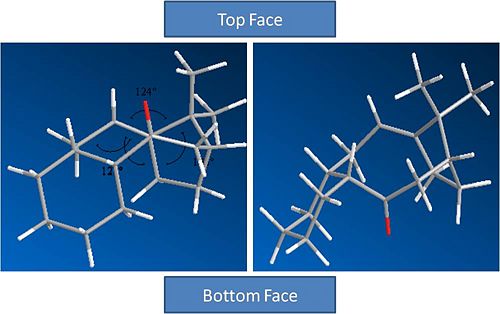
Molecule 10:
Isomer 1 |
Molecule 9
Molecule 10Analysis and Results
As discussed previously in the pyridinium calculations, it is important to remeber there are many different geometries and conformations with a large range energies that could be generated. The energies generated for molecules 9 and 10 were achieved through many geometry optermizations and MM2 calculations. Manual edits were used to move the Carbonyl and to change the cyclo hexane ring to the preferred chair conformation. The Energies are shown in the result tables below.
| Energy of Molecule 9/Kcal/mol | Energy of molecule 10/Kcal/mol |
|---|---|
| 48.88 | 44.29 |
| Bend Energy/Kcal/mol | Stretch Energy/Kcal/mol | Torsion Energy/Kcal/mol | VDW Energy/Kcal/mol |
|---|---|---|---|
| 15.84 | 2.69 | 18.16 | 12.64 |
| Bend Energy/Kcal/mol | Stretch Energy/Kcal/mol | Torsion Energy/Kcal/mol | VDW Energy/Kcal/mol |
|---|---|---|---|
| 10.69 | 2.55 | 19.77 | 12.55 |
It can be seen from the results that the lowest energy isomer is molecule 10. Both molecules adopt a chair conformation in the cyclo hexane ring. In molecule 9 the carbonyl sits on the top face (see picture above). This leads to poor overlap between the C=O sigma* orbital and the sigma C-H bonds adjacent to it due to poor geometry. In molecule 10 there are two C-H bonds anti peri planar to the C=O bond. This leads to good overlap between the C=O sigma* orbital and the C-H sigma bonds. This will stabalise the molecule and lead to a thermodynamically favourable product.
This is illustrated below:
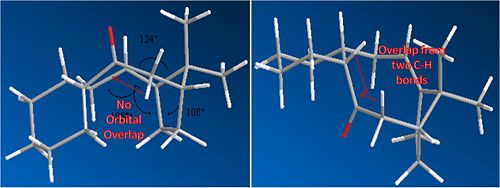
Alkene reactivity
The Alkene in these intermediates has been shown to be very stable and resitant to Hydrogentation. This can be shown by modelling the hydrogenated product. The model and results are shown below.
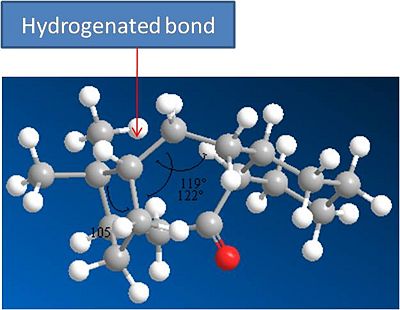
Hydrogenated Molecule 10:
| Energy of Molecule 9/Kcal/mol | Energy of hydrogenation product/Kcal/mol |
|---|---|
| 44.29 | 53.03 |
| Bend Energy/Kcal/mol | Stretch Energy/Kcal/mol | Torsion Energy/Kcal/mol | VDW Energy/Kcal/mol |
|---|---|---|---|
| 10.69 | 2.55 | 19.77 | 12.55 |
| Bend Energy/Kcal/mol | Stretch Energy/Kcal/mol | Torsion Energy/Kcal/mol | VDW Energy/Kcal/mol |
|---|---|---|---|
| 13.47 | 2.83 | 22.05 | 15.45 |
Analysis:
It can be seen that the hydrogenated product has a significantly higher energy than that of molecule 10. A break down of the energies reveals that the hydrogenated product has a significantly higher bend and torsion energies. This can be explained through analysing the bond angles of the newly formed sp3 hybridised carbons. The preferred bond angles for an sp3 carbon are 109 degrees. if we examine this molecule (shown above) the bond angles are 119 and 122 degrees. This is much larger than that of an sp3 carbon and is infact much closer to an sp2 hybridisation i.e the hybridisation found in an alkene bond. Thus the resulting hydrogenation of molecule 10 destabalises the 10 membered ring and explains why the alkene is very stable.
This type of alkene is also known as a hyper stable alkene or bridge head alkene. This concept employs the concept of Olifin Strain Energy (OSE). (see litrature below). OSE refers to the strain energy of the alkene minus the strain energy of the resulting alkane after a hydrogenation. For most cyclic alkenes this value is postive i.e. the alkene is more strained than the alkane. A good example would be Cyclo Hexene and its hydrogenation product Cyclo Hexane. however a hyper stable alkene refers to a situation where the OSE is negative i.e. The alkene is less strained than the alkane. Molecules 9 and 10 exhibit this and hence are reffered to as hyper stable alkenes.
References:
stability of bridgehead olefins http://pubs.acs.org/doi/abs/10.1021/ja00398a003
Section 2: Modelling Useing Semi Emperical Molecular Orbital Theory
As discussed previously the Molecular Force Field Method (MM2) provides good approximations for the energies of molecules, however it does not take into account the electronic properties of molecules. To further improve modelling techniques, Semi emperical methods have been developed to take into account electronic contributions.
The main method involves the Hartree-Fock algorithem. This is typically used to solve the time idependent Schrodinger equation for a multi electron atom or molecule. Understandably this is a complex problem to solve, and is hence solved numerically through the use of iteration.
The algorithem takes into account 5 major approximations:
1.The Born-Oppenheimer approximation (the molecular wave function is a function of the coordinates of each nuclei)
2.Relativistic effects are ignored
3.The variational solution is assumed to be a linear combination of finite basis orbitals
4.The energy of each eigen function is assumed to be a single slater determinant
5.The mean field approximation is applied
All these approximations lead to errors in the calculations. More complex methods have been developed such as the density functional theory. However this will not be used for this exercise.
Modelling Regioselective Addition of Dichlorocarbene
The molecule shown below is to be modelled:
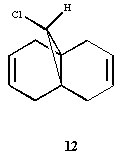
This molecule has been shown to be regio selective towards electrophilles. The alkene which is syn to the chlorinre reacts with electrophilles rather the alkene which is anti to the chlorine. From initial inspection this seems strange, as the syn face is much more hindered than the anti face, as chlorine is a large atom.
Through applying the Hartree-Fock algorithem (the MOPAC PM6 method on ChemBio3D shall be used) and producing the molecular orbitals of this molecule, an explanation for this selectivity shall be generated. Further analysis of the MO's will also reveal which alkene is more reactive to nucleophilles.
Results:
MM2 results:
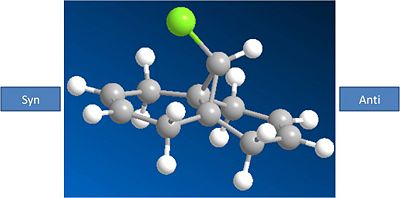
Molecule 12 MM2 Optimized:
| Total Energy/Kcal/mol | 17.897 |
| Stretch Energy/Kcal/mol | 0.63 |
| Bend Energy/Kcal/mol | 4.69 |
| VDW Energy/Kcal/mol | 5.80 |
| Torsion/Kcal/mol | 7.68 |
MOPAC Results:
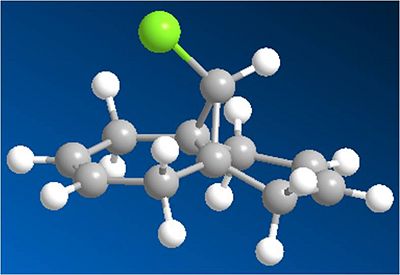
Molecule 12 MOPAC Optimized:
| Total Energy/Kcal/mol | 19.74 |
Analysis:
It can be seen that the Total energy of the molecule increases after the Hartree-Fock algorithem has been applied. It can also be seen that the geometry has changed. By applying simple geometry to the molecule it can be seen that the distance between the chlorine and the syn alkene has increased in the Hartree-Fock optimised molecule.

| MM2 Optimised/A | MOPAC Optimised/A |
|---|---|
| 3.03 | 3.23 |
Through the Hartree-Fock method the MO's of the molecule can be generated graphically. The MO's to be modelled are the Highest Occupied Moleculare Orbital (HOMO), the Lowest Unoccupied Molecular Orbital (LUMO), the HOMO -1 and the LUMO +1. These are shown below. Note the isocounter has been used to adjust the graphical MO's in order to show the relevant orbitals.
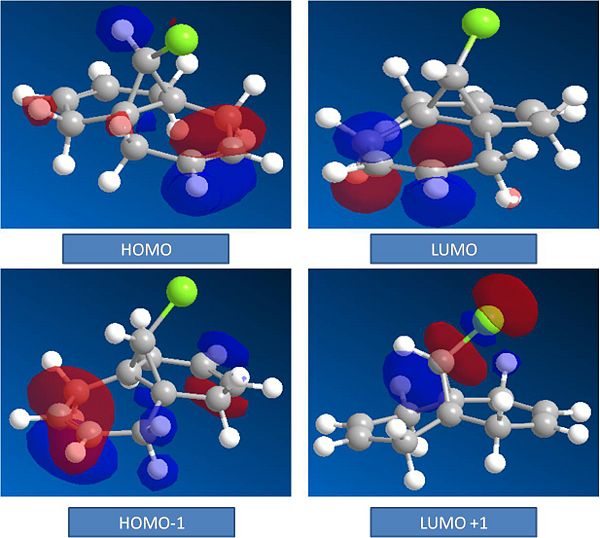
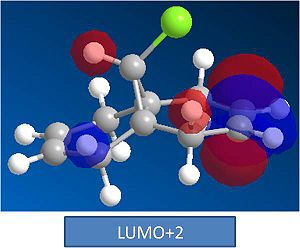
It can be seen from the MO's that the HOMO is found on the syn Alkene. This explains the observed reactivity to electrophilles. The LUMO is found on the anti alkene. This LUMO is lower in energy than the Anti bond on the syn alkene which is observed at LUMO+2. From this it can be concluded that the anti alkene is more reactive towards nucleophilles than the syn alkene.
Calculating the influence of the C-Cl bond on the vibrational frequency of the molecule
In this exercise the effects of the anti alkene bond on the vibrations of the C-Cl bond. This will be achieved through comparing the stretching frequencies of these bonds in molecule 12 to the stretching frequencies of a hydrogenated product (where the anti alkene has been hydrogenated).
This shall be achieved through using ChemBio3D and running MM2 and MOPAC calculations on the hydrogenated product saving the results for this molecule and molecule 12 as a Gaussien input file. This shall then be sent to a very large computer which will calculate the vibrational frequencies and intensities. The file shall then be opened in gauss view. Gauss view can then generate a predicted IR and from this the streching frequencies of the alkene double bonds and the carbon chlorine bond can be determined.
Results
The results for Molecule 12 are shown below:
IR Spectra:

Anti Alkene stretch:

Syn Alkene Stretch:
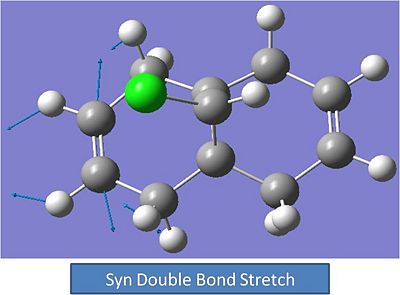
Carbon-Chlorine Stretch:
| Bond | Stretching Frequency/cm^-1 |
|---|---|
| Anti C=C bond | 1737.01 |
| syn C=C bond | 1757.43 |
| C-Cl bond | 770.79 |
Results for the the hydrogenated anti alkene:
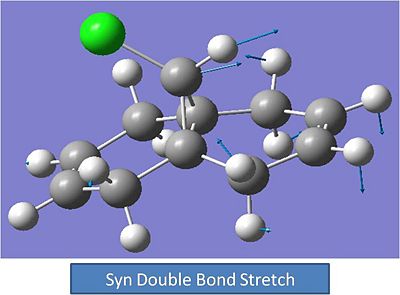
IR Spectra:
Anti C-C Stretch:

Syn Alkene Stretch:

Carbon-Chlorine Stretch:
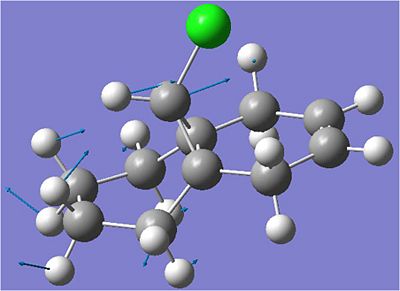
| Bond | Stretching Frequency/cm^-1 |
|---|---|
| C-C bond | 1393.0 |
| syn C=C bond | 1753.76 |
| C-Cl bond | 779.92 |
Analysis:
From the results it can be seen that the stretch of carbon-chlorine bond has increased in wave numbers in the hydrogenated molecule. This effectivly means that the bond has increased in length. The differnce in the stretches is roughly 9cm^-1.
This can be explained by considering the sterio electronics and the molecular orbitals of molecule 12. When there is an alkene anti to the chlorine, it allows overlap between the carbon-carbon pi bond and the carbon-chlorine sigma* bond. This stabalises the molecule, however will destabalise the carbon chlorine bond and in turn increase the length of it. This explains the increase in wave numbers.
In comparison to the molecule with the carbon single bond anti to ths chlorine, there will be no overlap with the sigma*. this means the nond length will be shorter.
This result is also expected from the MO diagrams, and results from an overlap of the HOMO -1 and the LUMO +1. These were shown above.
Mini Project:Diaryl Silyl Ether as Catalyst of an Exo selective, Enantioselective Diels-Alder Reaction
Introduction:
This project will look into modelling the peoducts of an Exo Selective Diels-Alder reaction. As discussed in the section on cyclo pentadiene dimers, the preferred product of a Diels-Alder reaction is the Kinetic Endo product. This is due to the endo rule which states that the endo transition state is lower in energy than the exo transition state and hence the preffered product is the endo product, even if this is thermo dynamically more unstable than the exo product.
The Synthesis above is interesting as the thermodynamic exo product is formed, plus the reaction is enantioselective. The reaction being modelled involves an organic iminium catalyst. More specifically a catalyst which is very similar to Jorgenen's Catalyst. This has been describbed as an "obese" catalyst, and is availiable from sigma aldrich.
The catalyst is useful as it producces only 1 enantomer of both the Endo and Exo product. This means that only two products are formed rather than a potential 4. The reaction being modelled involves an unsaturated aldehyde (an eanal), cyclo pentadiene, TFA (which is a week acid) and the catalyst. The reactionpromotes the formation of an iminium ion between the Aldehyde and the Catalyst. Due to the truely vast size of the catalyst it hinders certian faces of the reacting dienophille. This results in a steio selective Reaction.
Reaction Scheme:

The best way to show the reasons for the exo and enatioselective selectivity is through modelling the catalyst and the the iminium ion and determining the hindered faces. This is shown below.
A reaction such as this would be very useful when forming a new synthesis for new molecule.
The reaction does not yeild 100% exo product, and a mixture of of both the exo and endo isomers will be made. When these new molecules are made it is difficult to prove which isomer is which. The main methods involved in analysing molecules are Infra Red spectrosciopy, H1 NMR, C13 NMR and we can look at the Optical Rotation.
Modern computational techniques can produce predictions of these spectra and properties. This would help in determining which isomer is the major product, and in a reaction such as the one above would aid in proving if the catalyst had worked or not.
The aim of this project is to model the spectra and and properties of the Exo and Endo products of the above reaction and to explain the regio selectivity of the reaction. The results shall then be compared to literature results. From this conclusions can be drawn on how accurate and useful modern computational techniques are at predicting spectra and properties of molecule.
Models of Catalyst and Imidium Cat Ion:
Catalyst: Energy = 41.66Kcal/mol
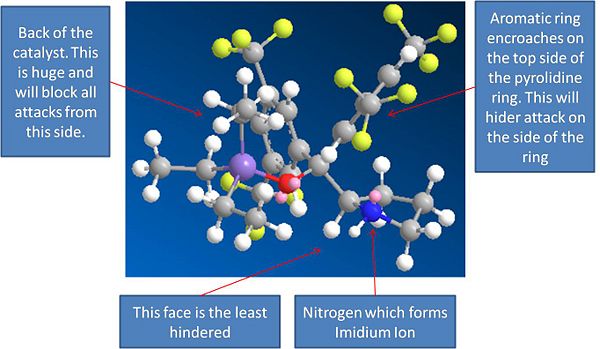
Catalyst
Iminium cat Ion:Energy = 42.01Kcal/mol
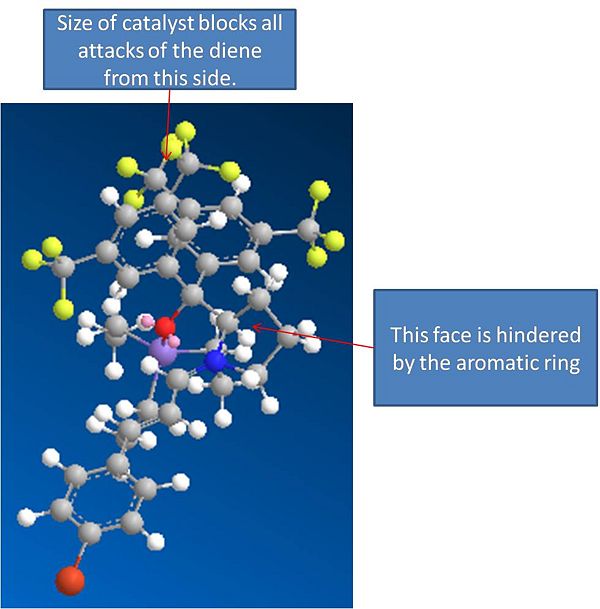
Iminium cation
Analysis of Models:
The MM2 method was used to minimise the energies of these molecules. It can be seen from the models that when the catalyst reacts with the aldehyde it completely blocks off one side of the alkene, acting a bit like a molecular "wall". This will prevent attacks from this side due to sterics, and hence explains the enantioselectivity of the reaction, as the cyclo penta diene can now only attack from one side.
Initially models of the products with the iminium cation still attached (before it is cleaved by water in an acid catalysed reaction to produce the final product) were modelled to see if the exo selectivity could be explained through the energies of these transition states. The mosels and results are shown below. Please note that these molecules are very large and cannot be accuratly viewed in a picture, so please see the jmols.
Exo Product and Iminium Cat Ion: Energy = 85.42Kcal/mol
Exo Product and Iminium Cat Ion:
Endo Product and Iminium Cat Ion: Energy = 85.76Kcal/mol
Exo Product and Iminium Cat Ion:
From the models it was determined that the exo transition state was lower in enegy however only by 0.34Kcl/mol. This to small to draw any conclusions.
It was decided to return to the sterics arguement to determine the Exo selectivity of the reaction.
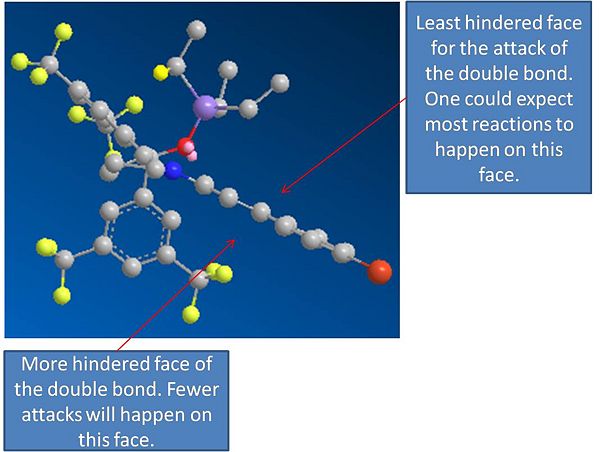
From the model of the iminium cat ion, it can be seen that one side is completly blocked from attack, and that on face of the alkene is more hindered. This was shown above in the picture of it. If we consider the attack of cyclopentadiene on the side of the molecule that is not blocked, and on the face that is less hindered we can model the following reactions:
Note: both reactions are 4n+2 Thermal. This means they are supra facial and lead to Dis rotation.
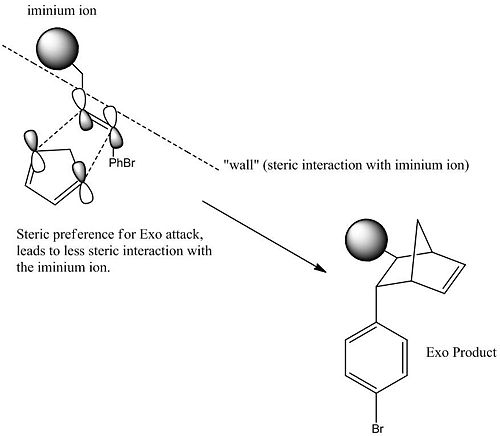

From ananlysis of the molecules it has been determined that sterics control the the path by which the cyclopentadiene can attack. The most favourable attack is the one that favours the steric prefernce for the exo attack, as there is less steric interaction with the iminium ion. The cyclopentadiene could attack from the other side of the double bond, however this is ore sterically hindered and will not happen as often. When it does happen, it will lead to the formation of the endo product.
Products to be Investigated:
With the modelling of the transition states complete and the reaction selectivity explained, the reaction products can now be modelled and there Spectra and properties predicted. The products were modelled on ChemBio3D. Their geometries were optimised using the Molecular Force Field MM2 Method and then optimised further using gaussien. The models and Energies are shown below.

Endo Product:
Exo Product:
| Exo Product/Kcal/mol | Endo Product/Kcal/mol |
|---|---|
| 31.53 | 29.89 |
| Exo Product/a.u. | Endo Product/a.u. |
|---|---|
| -3188.2425 | -3188.243 |
Analysis:
In the MM2 calculation the endo product is thermo dynamically more stable than the exo product. After the geometry had been optimised using gaussien, it was found that both products are pretty much identical in energy. This is further proof that the reaction is governed by steric factors.
The next step is to see if computational techniques can predict the spectra of these molecules. This can be achieved through predicted the NMR on gaussien and then comparing the results to literature. If the results are positive, then it will demonstrate that you can predict an NMR before forming the product and thus determine the major product Quickly.
C13 NMR:
The first spectra to be predicted will be the Carbon 13 NMR. the important information to take from the carbon 13 NMR are the chemical shifts. The intensities of the spectra have no real meaning, as the relaxation time of carbon is very long and dependent on the groups that surround it. However the intensities of the peaks could could show which product is the major product, as one would expect the peaks of the major product to be more intense.
The literature carbon 13 nmr was run in CDCl3 and was run as a mixture of both Endo and EXO products. The data and spectrum shown below:
Literature Carbon 13 NMR and Data:
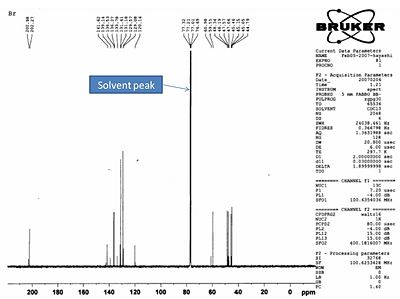
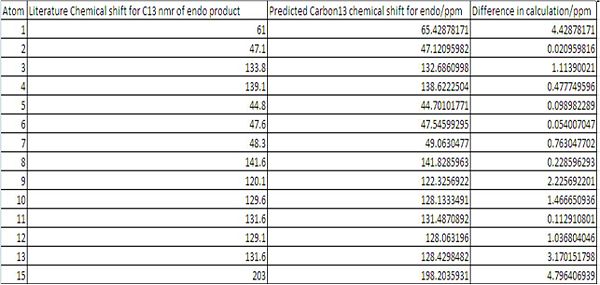
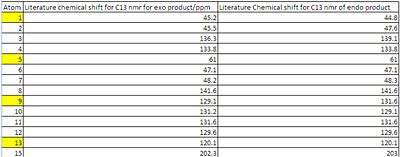
13C-NMR δ/ppm 203.0, 202.3, 142.7, 141.6, 139.1, 136.5, 136.3,133.8, 131.6, 131.2, 129.6, 129.1, 120.1, 120.0, 61.0, 59.6, 48.3, 48.2, 47.6, 47.1, 45.5, 45.2,45.1, 44.8
Predicted Spectra: Results for Exo NMR

Data Analysis
Predicted Spectra: Results for Endo NMR
Data Analysis:
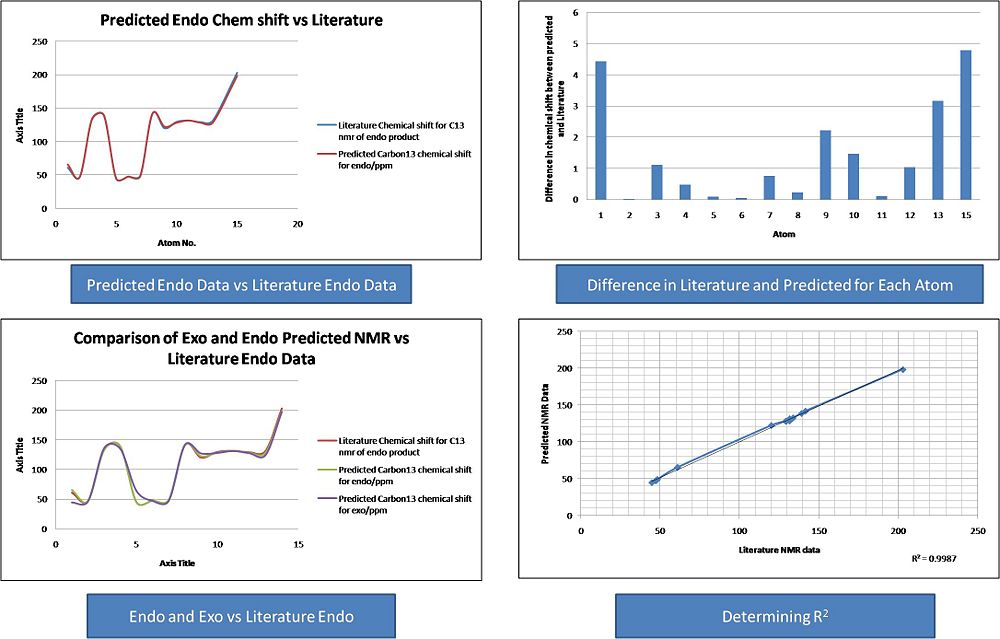
Analysis of Carbon 13 NMR results:
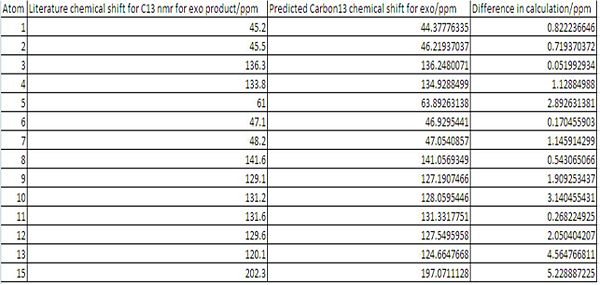
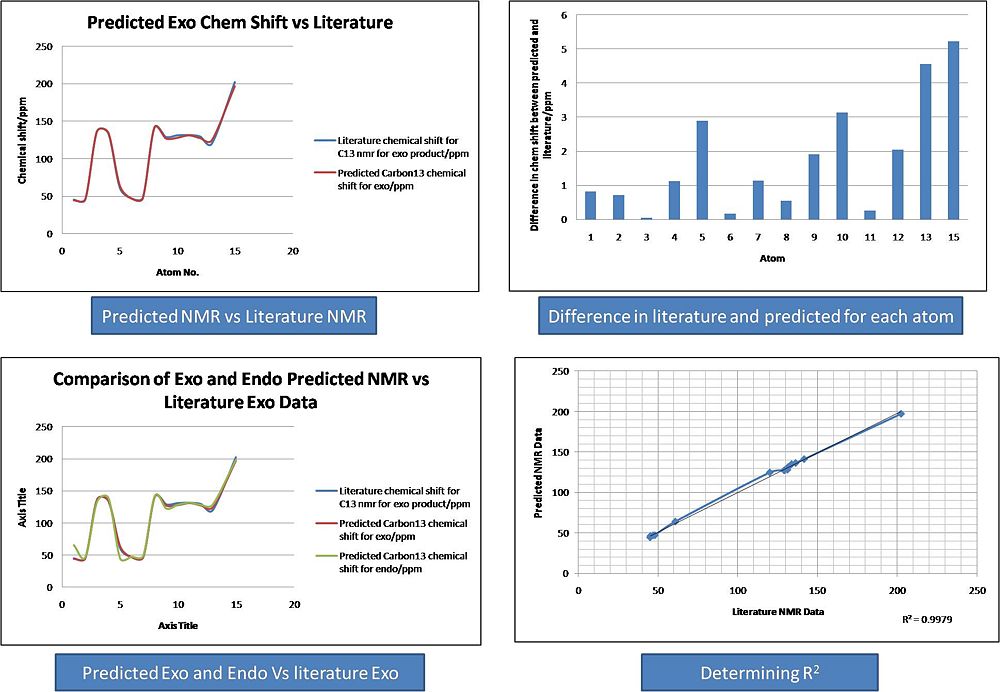
It can be seen from the Results that both the Endo and Exo NMR's fitted the Literature Carbon 13 NMR very well. A plot of Literature Chemical Shift against predicted Chemical Shift was produced for each NMR. This was done in order to determine the R2 value. It was found that the endo that both NMR's fitted the literature value with R2 values of 0.99, implying a excellent fit between the data. This was also shown graphically by plotting the literature Exo vs the predicted Exo and by plotting the literature Endo vs the predicted Endo.
Despite the very strong fit between the data, there were still shifts that do not corelate exactly to the literature. The worst atoms for correlating to the literature are:
Exo Atoms: 5, 10, 13 and, 15
Reasons for the discrepencies could be:
5: Is Adjacent to the carbonyl group. The the carbonyl group is known to have an error associated with the calculation due to errors in the spin orbit coupling. this could also effect carbon number 5.
10 and 13: the positions 10 and 13 find themselves in in the opptimal geomerty are more shielded/deshielded than the positions 9 and 12 find themselves in. Experimentally there will be free rotation around the carbon bond between 1 and 8. To improve these results an average of 13 and 10 and 9 and 12 should be taken and compared to the literature.
15: Atom 15 is the carbonyl carbon. This suffers from inaccuracies in the calculations due to errors in the spin orbit coupling. to correct for this the predicted value should be multiplied by 0.96 and have 12.2 added to it. This would bring the predicted value of the Chemical shift to 201.4ppm this is much closer to the literature chemical shift of 202.3ppm.
Endo Atoms: 1, 9, 13 and, 15
1:Is Adjacent to the carbonyl group. The the carbonyl group is known to have an error associated with the calculation due to errors in the spin orbit coupling. this could also effect carbon number 1.
9 and 13: the positions 10 and 13 find themselves in in the opptimal geomerty are more shielded/deshielded than the positions 9 and 12 find themselves in. Experimentally there will be free rotation around the carbon bond between 5 and 8. To improve these results an average of 13 and 9 and 10 and 12 should be taken and compared to the literature.
15: Atom 15 is the carbonyl carbon. This suffers from inaccuracies in the calculations due to errors in the spin orbit coupling. to correct for this the predicted value should be multiplied by 0.96 and have 12.2 added to it. This would bring the predicted value of the Chemical shift to 202.47ppm this is much closer to the literature chemical shift of 203ppm.
The NMR's of the two compounds are very similar. Through plotting both the NMR' on the same graph it is possible to see which atoms have different chemical shifts. Atoms 5 and 1 in the exo product appear different to atoms 1 and 5 in the endo product. Effectivly the chemical shifts have switched positions and this follows the switch in the carbonyl group and the aromatic group. If these atoms are relabeled in the endo product, then the NMR's look identical. This is shown below:

It can bes seen that there are small differences between the spectra , but these are not large enough to draw any real conclusions from.
Conclusion of NMR:
It can be concluded that it is not possible to tell the difference between the NMR's and thus it is impossible to determine the different isomers from the Carbon 13 NMR for these products.
Predicting IR Spectra:
The IR spectra of the Exo and Endo products shall be modelled to see if there are any differences between the two. These shall then be compared to literature to see if the Isomers are different.
literature IR:
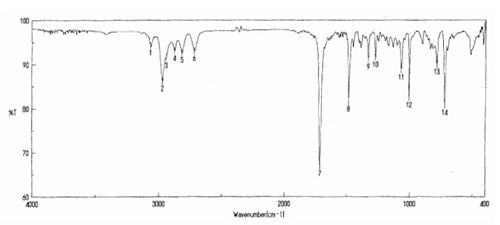
IR (neat) ν 3066, 2971, 2941, 2876, 2816, 2718, 1716, 1489, 1009, 726 cm-1
Predicted Exo IR:
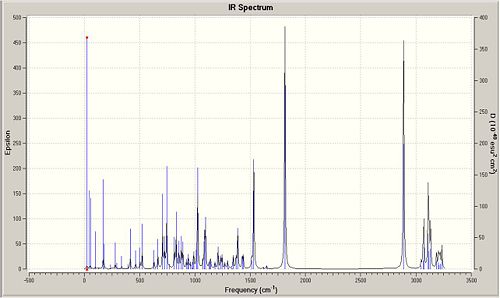
Predicted Endo IR:

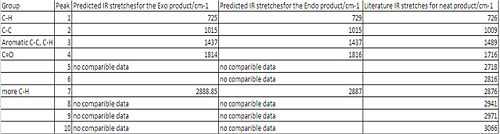
Results of IR modelling:
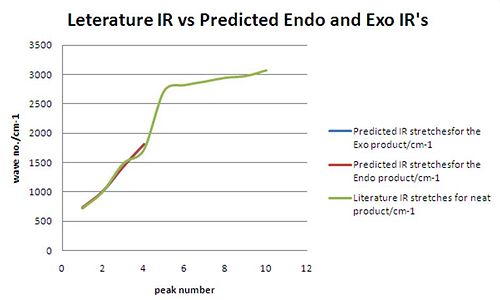
Analysis of IR modeliing:
The results for the predicted IR spectra are poor. It can be seen from the results that there is a good comparison to literature for low wave numbers however there were no comparible results for wave numbers higher than 1814cm-1. The Predicted IR spectra's are known to have errors in them, as the bond frequencies are calculated in the gas phase. it the lab this is not always the case, and the IR's are calculated in the solid or liquid phase. This instantly means the results are not directly comparible.
The carbonyl stretch is the most notible peak. this was observed at 1714cm-1 in the literature. The predicted IR's preicted them at 1814 and 1816cm-1. This is 100cm-1 greater than the literature value.
Again the difference in the Endo and Exo Ir's are to small to draw any conclusions as the predicted sttretches are to similar.
Conclusion to Project:
This project has looked into explaining the selectivity of a given reaction and into modelling and predicting the IR and C13 NMR spectra of the products.
The project was sucessful in explaining the selectivity of the reaction. For this molecular mechanics methods were used to optimize the geometries of key transition states. Then steric factors were taken into account to form a reasonable explaination for the regio selectivity.
However the project was less sucessful in determining the major and minor products of the reaction from predicted spectra. While the predicted NMR fitted the literature NMR, the differences in the Exo and Endo products NMR's were too small to produce any conclusions. Again the predicted IR's fitted the the Literature IR for low wave numbers, however a lack of applicable data at high wave numbers meant that the IR did not reveal any significant differences in the spectra and hence, did not help in determining the major or minor product of the reaction.
In the journal associated with the project, a chiral column was used to seperate the two products. Further investigations into the optical rotation of the products could reveal the the missing piece of the puzzle. This could be modelled using a computational technique on gaussview and then compared to a literature value, however this was not given in the journal.
References:
Main Jouranl: http://pubs.acs.org/doi/suppl/10.1021/ol071009%2B
Iminium Catalysis: http://pubs.acs.org/doi/abs/10.1021/cr068388p
Jorgensen's catalyst: http://www.sigmaaldrich.com/chemistry/chemical-synthesis/technology-spotlights/jorgensen-organocatalysts.html
