Miguelmod article
NH3
Summary
| Calculation method | RB3LYP |
| Basis set | 6-31G(d.p) |
| Final energy | -56.55776873au |
| RMS gradient | 0.00000323 |
| Point group | C3V |
| Optimised bond distance | 1.01798Å |
| Optimised bond angle | 105.745 |
Item Value Threshold Converged?
Maximum Force 0.000006 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000014 0.001800 YES
RMS Displacement 0.000009 0.001200 YES
Predicted change in Energy=-1.141602D-10
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.018 -DE/DX = 0.0 !
! R2 R(1,3) 1.018 -DE/DX = 0.0 !
! R3 R(1,4) 1.018 -DE/DX = 0.0 !
! A1 A(2,1,3) 105.7446 -DE/DX = 0.0 !
! A2 A(2,1,4) 105.7446 -DE/DX = 0.0 !
! A3 A(3,1,4) 105.7446 -DE/DX = 0.0 !
! D1 D(2,1,4,3) -111.8637 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
File:MN1315 NH3CORRECTED OPT.LOG
NH3 |
Frequency Analysis
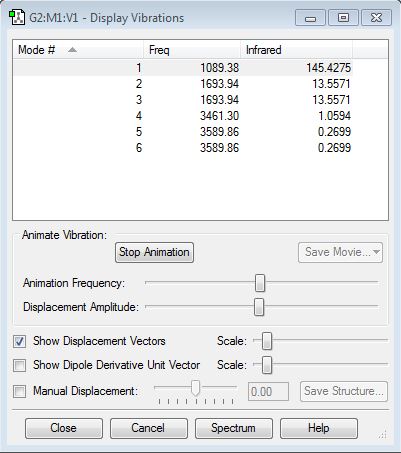
From the 3N-6 rule I expect 6 vibrational modes. Modes 5 and 6 are degenerate, as are 2 and 3. Modes 1, 2 and 3 are "bending" vibrations and modes 4,5 and 6 are "bond stretching". Mode 4 is highly symmetric, mode 1 is the "umbrella" mode and I would expect to see 3 bands in an experimental spectrum of gaseous ammonia.
Charge Analysis
The charge distribution generated assigned charges of 0.375 to the Hydrogen atoms and a charge of -1.125 to the Nitrogen atom. This aligns with my expectations - Nitrogen is the more electronegative atom.
H2
Summary
| Calculation method | RB3LYP |
| Basis set | 6-31G(d.p) |
| Final energy | -1.17853936au |
| RMS gradient | 0.00000017 |
| Point group | D*H |
| Optimised bond distance | 0.74279Å |
| Optimised bond angle | 180 |
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
Predicted change in Energy=-1.164080D-13
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 0.7428 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
File:MN1315 H2CORRECTED OPT.LOG
H2 |
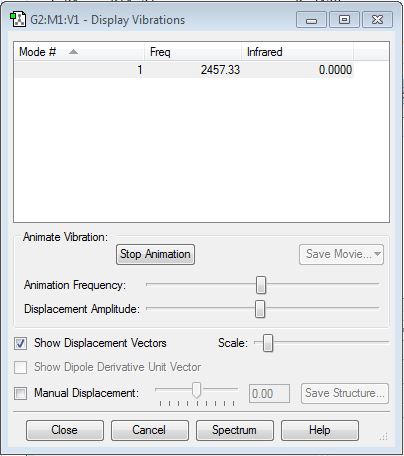
Frequency Analysis
N2
Summary
| Calculation method | RB3LYP |
| Basis set | 6-31G(d.p) |
| Final energy | -109.52412868au |
| RMS gradient | 0.00000060 |
| Point group | C3V |
| Optimised bond distance | 1.10550Å |
| Optimised bond angle | 180 |
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
Predicted change in Energy=-3.401036D-13
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.1055 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
N2 |
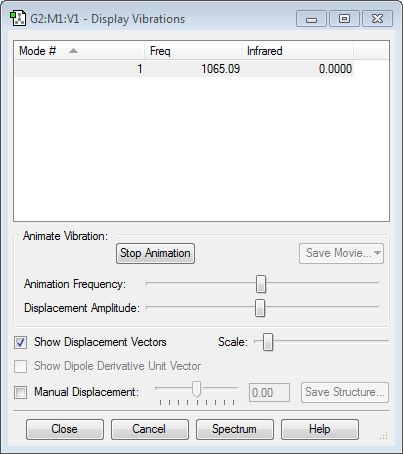
Frequency Analysis
Reactivity
What is the energy of the reaction N2 + 3H2 -> 2NH3?
E(NH3)= -56.55776873au
2*E(NH3)= -113.11553746au
E(N2)= -109.52412868au
E(H2)= -1.17853936au
3*E(H2)= -3.53561808au
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= 0.0557907au = 146.47848285kJ/mol
F2
Summary
| Calculation method | RB3LYP |
| Basis set | 6-31G(d.p) |
| Final energy | -199.49825218au |
| RMS gradient | 0.00007365 |
| Point group | D*H |
| Optimised bond distance | 1.40281Å |
| Optimised bond angle | 180 |
Item Value Threshold Converged?
Maximum Force 0.000128 0.000450 YES
RMS Force 0.000128 0.000300 YES
Maximum Displacement 0.000156 0.001800 YES
RMS Displacement 0.000221 0.001200 YES
Predicted change in Energy=-1.995024D-08
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.4028 -DE/DX = 0.0001 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
N2 |
Frequency Analysis
Charge Analysis
This is a neutral molecule.
Molecular Orbitals
The 2s AOs on each F atom contribute to this sigma bonding, unoccupied MO.
The 2s AOs on each F atom contribute to this sigma* anti-bonding, unoccupied MO.
The 2p AOs on each F atom contribute to this sigma bonding, unnoccupied MO.
The 2p AOs on each F atom contribute to this pi bonding, unoccupied MO.
The 2p AOs on each F atom contribute to this pi bonding, unoccupied MO. It is in a plane which is perpendicular to that of the above pi bonding MO.