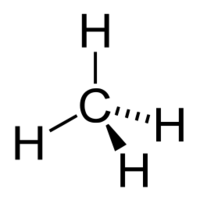
Methane
Introduction
Methane is the simplest carbon hydrogen structure, and one of the most abundant ones found in nature, being widely produced by many types of livestock, and found in gasfields. It is a colourless, odourless gas, which has a tetrahedral shape, and a bond angle of 109.5°. The strength of the carbon hydrogen covalent bond in methane is among the strongest in all hydrocarbons. It can undergo several reactions, including combustion and halogenation.
Reactions of Methane
Combustion
Methane can undergo full or partial oxidation:
Partial:
Full:
3D structure of methane
| Methane | |
|---|---|
| |
| General | |
| Systematic name | Methane |
| Other names | Natural gas, marsh gas, firedamp |
| Molecular formula | CH4 |
| SMILES | C |
| Molar mass | 16 |
| Appearance | Colourless gas |
| CAS number | 74-82-8 |
| Properties | |
| Density & phase | 0.717 kg/m³ gas |
| Solubility in water | 3.5 mg/100 ml (17°C) |
| Melting point | 90.6 K |
| Boiling point | 111.55 K |
| Structure | |
| Molecular shape | Tetrahedral |
| Coordination geometry |
tetrahedral |
| Dipole moment | 0 D |
| Hazards | |
| MSDS | External MSDS |
| Main hazards | Highly flammable |
| Flash point | -188°C |
| R/S statement | R: R12 S:(S2) S9 S16 S33 |
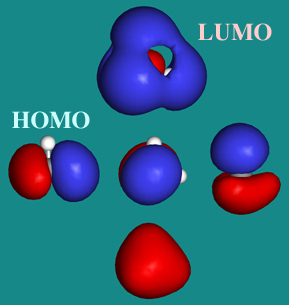
HOMO - LUMO Molecular Orbitals
Effects on Global Warming
Methane has been shown to be a more potent greenhouse gas than carbon dioxide (it retains up to 20 times more heat that carbon dioxide), but at the moment it remains at a much lower level than carbon dioxide. However this could change as time goes on, as landfills, increased livestock production, coal mining and rice cultivation all contribute heavily to methane production, and with ever expanding populations the demand for these comodities will only increase, raising the production of methane.