Mesyltoe 3O3
3.O3 Polymers: The Essential Guide
This is a summary of a lecture course given by Dr Joachim Steinke in Autumn 2012
Section 1
Polymer Terminology
Some definitions:
- Radical initiator: a radical species needed to start a polymerisation by reacting with a monomer (usually containing a vinyl group).
- Monomer: a polymer is a series of monomer repeat units.
- Initiation: the first step in a chain reaction.
- Propogation: the second step in a chain reaction where a polymer radical reacts with more monomer to form a longer chain.
- Termination: the final step in a chain reaction, which ends the polymerisation.
- Reactive Chain end: the propogating end of an active polymer chain, which can react with a monomer to extend the chain length.
- Repeat unit: the repeating structural motif in a polymer chain, usually identical to the monomer structure.
- Chain end: what it says on the tin, the end of a chain. If both chain ends have a functional group (distinct from the monomer) the polymer is telechelic. If only one of the ends is modified with a FG it is called a macromonomer.
- Backbone or main chain: the atoms that join the monomers together.
- Sidechain or pendant groups: structural motifs attached to the backbone.
- Random coil: this is a conformation of a polymer chain. If free rotation in the backbone is possible, the polymer adopts a random coil average structure (as all carbon centres can have many different conformations)
- Macromonomer: a macromonomer is a polymer chain containing one (or more) functional groups that can be polymerised further.
- Molar mass distribution: during polymer synthesis a distribution of molecular weights is made (usually).
Polymer Structures and Properties
Only highly symmetrical polymers can pack into a crystalline structure. Most polymers have crystalline regions and amorphous (or random coil) regions. Crystalline polymers are opaque and brittle. Semi-crystalline polymers can be either transparent or opaque depending on the size of the crystalline regions, and tend to be mechanically strong providing the amorphous region is above its glass transition temperature. Amorphous polymers with no chromophores will be transparent and colourless (and usually brittle).
Polymers have different properties to small molecules. Polymer chains tangle, and have large intramolecular forces because of their high 'surface area'. They take a very long time to move because they're so much bigger and heavier. The conformation space is vast because of the number of permutations in conformation possible.
Polymers have lots of properties that make them super uesful, including mechanical strength, elasticity, plasticity, thermal stability and very tunable properties.
To tune these properties, we create different structures. There are linear, telechelic and macromonomer polymers, all of which we've already met. There are also branched polymers, brush or comb polymers (long, regular and frequent branching), network polymers (lots of branching and irregular interconnection between backbones), hyperbranched (lots of branching and sub-branching from one backbone), and dendrimer (snowflake-like hyper-regular branching structure).
Most synthetic polymers are produced with a distribution of chain lengths leading to a Gaussian molecular weight distribution. Biopolymers or dendrimers can be monodisperse (have only one molecular weight present), but all others are polydisperse.
Polymers have complex solubility behaviour owing to the size of the molecules and the corresponding size of the the inter and intramolecular reactions. The time taken for them to diffuse also means that polymers take longer to dissolve than smaller molecules, sometimes a lot lot longer.
When polymers are being produced for commercial consideration, the viscosity of the polymer must be balanced with the weight of the polymer. The longer the chain length, the higher the viscosity, and the more energy required to process the polymer. However, the longer the chain length, the better the mechanical properties.
There are four main classes of polymers, defined by their response to stress (for example, sustained weight application). Fibres show a very small change in length under stress as the crystalline regions provide crosslinks to keep the amorphous regions together. Energy can dissipate by converting crystalline regions to amorphous regions. Once there are no more crystalline regions to convert, and the amorphous regions can no longer stretch, the polymer will suddenly break. Rigid plastics are similar, but they have no crystalline regions to dissipate energy into, so the break (failure) is more dramatic. A flexible plastic initially behaves like a rigid plastic, but at a point of increasing strain the amorphous polymer reorganises, dissipating some of the energy. This changes the properties of the polymer irreversibly. An elastomer responds to stress immediately and at low stress values. Flexible (mobile) polymers dissipate energy by switching to higher energy conformations. Once it has reached the highest energy conformer, there is nowhere to dissipate the energy, and the polymer breaks like a rigid plastic. Before it breaks, the elastomer responds reversibly to stress.
Chain entanglement creates viscosity and elasticity in a polymer, but it is time dependent: left to gravity, the polymers will gradually untangle, and elasticity will disappear. Vulcanisation creates crosslinking between polymer chains, meaning that the entanglement is permanent, and that the polymer will hold its shape. The more crosslinking, the less elastic the polymer. In vulcanisation specifically the crosslinks are made of C-S-C bridges. Blood clotting works in a similar way.
Structure-Property-Temperature Relationship
When amorphous polymers melt they change from an amorphous solid state to a rubbery state and finally to a liquid (polymer melt). The transition from a glassy state to a rubbery state is called the glass transition temperature (Tg). Amorphous polymers do not have a true melting point. Crystalline polymers don't have a Tg, but do have a true melting point, Tm. Semi-crystalline polymers have both a Tm from the crystalline contribution and a Tg from the amorphous contribution. These temperatures can be measured by specific heat and volume changes. Crystalline polymers have a sharp change in volume and heat capacity. Amorphous polymers show a gradual change. Both kinds of polymer have a lower volume at lower temperatures.
The Tg of a polymer is dependent on the degree of rotational freedom around the backbone and the translational freedom of the chains t slip past one another. This is controlled by intramolecular conformational flexibility and intermolecular packing. In summary, the more flexible a backbone and the smaller and more felxible the sidechain, the lower the Tg. Bulky sidegroups close to the backbone increase Tg, but far from the backbone decrease it. Strong intermolecular interactions increase Tg.
Section 2
Polymer Mechanisms
Nature has many mechanisms for structurally precise polymers in aqueous solution at room temperature, eg. peptide, carbohydrates, polynucleotides. We can imitate this with automated polypeptide synthesis.
There are two main mechanisms for polymer synthesis: step-growth (growth from both ends) and chain-growth (growth on one end). The implications for molecular weight on each of these mechanisms is different.
We want to control in our syntheses:
- Degree of polymerisation (length of chain)
- Molecular weight distribution
- Polymer chain end groups
- Pattern of co-monomer incorporation
- Degree of branching
How much control there is over these properties is dependent on the synthesis mechanism used. Which mechanism is used depends on the monomer, and which monomer is used depends on the properties desired from the monomer.
Chain Growth Polymerisation
The polymer chain grows in order direction with monomer adding only to the reactive polymer chain end. The polymerisation is exothermic and very fast. The reaction is enthalpically driven by the loss of C=C and the formation of C-C bonds. The ratio of recombination (functional groups bonding) to disproportionation (one functional group oxidising the other) depends on the monomer, and affects the molecular weight distribution. Both of these are termination steps, but disproportionation results in a macromonomer. The molecular weight distribution is close to Gaussian.
It is possible to derive the kinetics of a polymerisation from the molecular weight distribution. Steady state kinetics are assumed. The degree of polymerisation depends on Rp (rate of propogation), Rt (rate of termination) and [I] (concentration of initiator). Also important is Rct (rate of chain transfer). At constant [I], . The average chain length is thus independent of time, as long as the rates don't vary. We can use all of this to estimate a chain length.
Where kd is the initiator decomposition rate constant, v is the chain length and f is the initiator efficiency. Efficiency of initiators tend to be in the region of 50%, due to solvent effects and side reactions. This equation means that it's possible to tailor the chain length, sort of.
Step-Growth Polymerisation
This is also knows as condensation polymerisation. The chain grows by combination of any two species (monomer or oligomers). This is typically a slow and endothermic reaction, and the reaction is driven by the removal of a volatile condensation product (such as water). The polymer chain grows from both ends, and a high molecular weight of polymer is produced only at high monomer conversions. There is usually no termination step. The limiting factor in polymer size is the solubility of the polymer: when the polymer drops out of solution, it ceases to grow any further.
The kinetics of step-growth have no initiation, activation or termination step. The degree of polymerisation is determined by the stoichiometry of functional groups, viscosity effects on reaction rate and the solubility of the growing polymer chain. No polymer is produced until 95% of monomer has been converted (to oligomer or whatever). Side reactions are less important here than in chain-growth.
Carothers' Equation
Crucial for the successful synthesis of high molecular weight polymers is the purity of the starting materials. The correct ratio of starting materials is vital if one or more monomers are being used. The degree of polymerisation can be calculated using Carothers' equation.
Degree of polymerisation (DP) is dependent on p, the probability of finding a reacted functional group. If the ratio of comonomers A & B were 1:0.9, for example, p would be 0.9, and the DP would be low. The closer p is to one, the greater the degree of polymerisation.
Characterising Polymers
Polymers can be characterised by:
- Light scattering (sensitive to 'particle' size)
- Viscometry (Mark-Houwink equation: η = KMα
- GPC
- MALDI-tof
Most characterisation techniques are the same for small molecules and polymers. Differences appear when it comes to molecular weight characterisation. These methods must be appropriate for polymers. Some, such as viscosity and light scattering, are unique to polymer characterisation, as small molecules don't have the properties necessary for this sort of measurement.
MALDI-tof is a mass spectrometry technique that was developed for proteins. The polymer is dissolved in a solution containing a small molecule matrix which can crystallise in the presence of the polymer, and is able to ionise the polymer chain upon irradiation with a particular wavelength of light. The laser heats the matrix molecules, which then transfer vibrational and rotational to all parts of the polymer along with positive charge. This allows the polymer to vapourise without being heated to temperatures great enough to cause bond cleavage (which would give inaccurate and low mass measurements).
GPC or gel permeation chromatography uses a separation mechanism to determine the molecular weight distribution of a polymer. Longer polymer chains have a larger hydrodynamic radius in solution than shorter ones. The dissolved sample is passed through a porous column material that contains an equal number of different pore sizes. This means that longer polymers travel through the column faster, as there are fewer diffusion paths for the molecules to take.
From either of these methods, we obtain a molecular weight distribution curve. This shows the number average molecular weight, Mn(ΣNiMi/ΣNi), the weight average molecular weight, Mw (ΣNiMi2/ΣNiMi), and the viscosity average molecular weight, Mvisc. Ni is the number of polymers with molecular weight Mi. Another important thing is the width of the distribution, the PDI (polydispersity index).
Chain growth shows a sudden rise in DP, which tails off once a steady concentration in radicals has been reached. Regardless of the percentage conversion, the weight distribution remains the same. Step-growth shows very low DP (oligomers) up to 90% conversion, and then shows a sharp increase up to 100%. The greater the percentage conversion, the higher the average weight distribution.
Section 3
Living Polymerisation
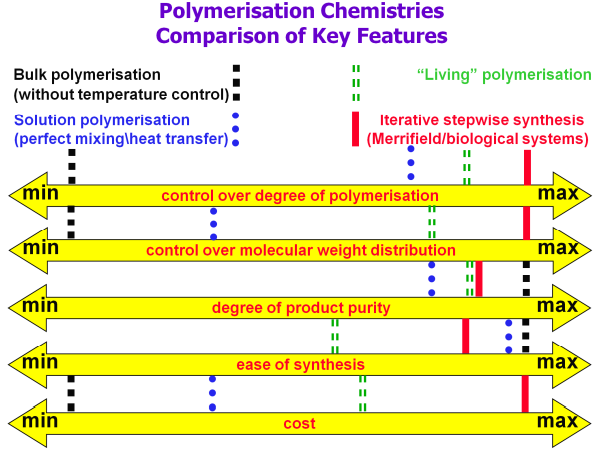
A living polymerisation is one that has initiation faster than propogation, no chain transfer and no chain termination. This means there is only one possible reaction pathway, and initiation is effectively instant.
Living anionic polymerisation is the best existing chemistry for very narrow molecular weight distribution polymers. Dynamic clusters of lithium ions act as a reversible protecting group. Alkyl lithium is a typical initiator. LiH s eliminated as a termination side reaction.
Dormant chain equilibria are clusters of charged species that do not initiate or propagate (thus are dormant). This prevents them taking part in side reactions. The proportion of the reactants in dormant chains depends on the solvent, concentration and temperature. This means that it can be optimised such that only polymerisation occurs.
Living polymerisation means that molecular weight grows linearly with % conversion (like in nature, hence living). It also means that block co-polymerisation is possible, where a second monomer is introduced partway through the synthesis. This allows immiscible polymers to be made miscible, and also allows creation of patterns on nm scale, for electronic devices.
In theory living polymerisations can be made from any polymerisation. The problem is developing chemistry that can stabilise the reactive chain end to prevent side reactions. This has been made possible.
In Group Transfer Polymerisation, a protecting group is transferred along the chain. The protecting group acts to deactivate the chains to prevent side reactions. In living Cationic polymerisation, like in living anionic polymerisation, clusters of charged species prevent side reactions. Cationic polymerisations, due to their larger active species, have proved harder to control. In living Free Radical Polymerisation, a stable free radical such as TEMPO combines with the C-centred radicals. The TEMPO-activator bond does not form as it is too unstable, even at room temperature. The TEMPO-monomer bond is thermally labile, allowing temperature to control the active proportion of reactant. Atom Transfer Radical Polymerisation using bromide as its protecting group. A copper catalyst that swiftly oxidises and reduces is used to capture and release bromide ions. Reversible Addition Fragmentation Transfer uses a reversible chain transfer to deactivate reactants. This chain transfer is very fast, faster than propogation. The chain transfer agent keeps switching between polymer chains.
Living Ring Opening Polymerisation can circumvent the limitation of high molecular weight not being accessible via step-growth. The release of ring strain is the driving force. This allows the production of polyesters via chain-growth, though the monomer production is more complex. Ring Opening Metathesis Polymerisation produces a metallacycle as an intermediate, and the driving force is relief of ring strain. This process can also be used to produce polymer precursors, which can be purified before the final polymerisation takes place.
For some monomers, no living polymerisation has yet been found. A means of molecular weight control is chain-transfer. Thiols are favoured because of their high CT efficiency, though they aren't as efficient as the CT agents used in RAFT. As a result, there is a maximum chain length they can possibly give, limited by kp/kt, as termination steps still occur. Catalysts can also be used as CT agents, and can act very similar to TEMPO. They react much faster than RAFT reagents. They also result in C=C bonds at the chain end, meaning the products are macromonomers that can, for example, be turned into polymer brushes.
Controlling Tacticity
To control the tacticity of our polymers, we can add bulky substituents that can be removed after polymerisation, use a chiral solvent that interacts with the monomer on the chain end in a particular fashion, or by using co-ordinating metal ions to predispose particular monomer configurations. In non-coordinating solvents, reactivity can be governed by coordinating ions, such as Li+, meaning that all groups approach from the same direction. In coordinating solvents, the approach is governed by sterics.
Summary
Oh, God, do we need a summary.
| Step | Chain | Living | |
|---|---|---|---|
| Polydispersity indices | 2 | 1.5-2 | 1.01 |
| How MW changes as reaction proceeds | Exponential increase | No change | Linear increase |
| Linear block copolymers possible? | Only with difficulty | No | Yes |
| Control over chain end functionality | Yes | Yes | Yes |
| Control over MW | Full control, lowish MW | Full control | Full control |
| Are high MW polymers possible? | No | Yes | Yes |
Section 4
Copolymers
A copolymer is an additional monomer used in polymerisation. They can be in many different structures. A diblock has a block of monomer A and a block of monomer B joined together. A triblock has a block of monomer B between two blocks of monomer A. A graft block has monomer B bonded onto a monomer A backbone, like a brush polymer. Alternating and random structures are self-explanatory. A tapered structure has A and B merging into one another, with a period of alternation in between. A dendro block is a dendrite with one half composed of monomer B. Alternating, random and tapered structures are caused by molecular blending, the two monomers being polymerised at the same time. Block, graft-block and dendro-block polymers have monomer B added onto monomer A after polymerisation.
In many cases, the properties of a homopolymer are insufficient for a particular application. Mixing polymers together is theoretically possible, but in practice entropy means that they tend to phase separate. Copolymers avoid this issue. The extent to which control over structure is possible depends on the mechanism.
In step growth polymerisation, the incorporation of different monomers is statistical. Block copolymers can be made by reacting telechelic homopolymers together. The reversibility of this kind of polymerisation means that there can be backbiting/scrambling between similarly reactive monomers, meaning isomerisation in the polymer. If the monomers have similar reactivity, random distribution is possible. If they have very different reactivites, then it is possible to make block copolymers. It isn't yet possible to control the molecular weight of these processes.
In chain growth (radical) polymerisation, the pattern of monomer incorporation is controlled by the electronic properties of the C=C bond and nucleo/electrophilicity of the the propagating polymer radical. Monomers with an electron rich and electron deficient C=C bond respectively produce alternative sequences. Monomers with C=C bonds of similar electron character give statistical copolymers. Tapered comonomer incorporation is possible with LFRP. The more reactive monomer will polymerise first, and then as it is used up, the less reactive monomer will polymerise. In chain growth (ionic and metal coordination) polymerisation, statistical incorporation of the monomers occurs.
Reactivity
The method with which a monomer can best be polymerised depends on the reactivity of the monomer, the nature of the polymerisable group and whether the functional groups are compatible with the chain or step-growth process. Monomers with electron rich C=C bonds work well with cationic and radical approaches. Monomers with electron deficient C=C bonds polymerise via anionic and radical mechanisms.
How to Choose Your Monomer
First, it must be identified whether the C=C bond needs to be electron rich or electron deficient. Next, we identify the possibility of radical or ionic chain transfer. Can this species form inductively or mesomerically stabilised radicals? Does the monomer have an acidic or basic site? Then we must consider the steric demands of the polymer. 1,2 disubstituted alkenes polymerise poorly, owing to the steric hindrance being greater than the enthalpic driving force.
It is theoretically possible to work out the co-reactivity of two or more monomers by calculating the frontier orbitals. In short, the more stabilisation energy gained, the more favourable the kinetics. I've never heard that one before...
Copolymerisation parameters
To avoid people having to figure out for themselves whether a copolymerisation will work or not, someone came up with the bright idea of inventing copolymerisation parameters, standards that people can measure. There are r1 and r2. The first is the ratio of rate constants of radical A reacting with A to radical A reacting with B. The second is the ratio of rate constants of radical B reacting with A to radical B reacting with B. Both being 1 gives an ideal polymerisation (random distribution). Both being less than one gives a statistical polymerisation. Both being greater than 1 gives a block copolymerisation. Both being equal to zero gives alternating polymerisation (radical B reacts only with A, and radical A only with B). If r1 >1 and r2<1, tapered copolymerisation results (A prefers to polymerise with itself, and B to copolymerise).
Graft Copolymers
The random coil conformation has implications on the reactivity in polymer grafting. It has vast steric bulk, and so can cause a lot of hindrance. However, its flexibility means that reactions can still occur. Using macromonomers, the graft is quite easy to control. It is possible, though rare, for the grafting to be so dense that only a few repeat units are possible, as is the case when the side chains are dendrimers.
There are three synthetic strategies for graft copolymers. 'Graft from' synthesised a linear polymer with side chain groups that can be initiating sites. The grafting reaction has the linear polymer dissolved in monomer 2, and polymerisation is initiated. In this synthesis, initiator efficiency is high, steric demand is low, termination reactions are low, reaction times are short, and initiation/propagation is high. The chain length is not well controlled.
The 'graft onto' approach synthesises a linear polymer with side chain groups that can couple to other functional groups. The grafting reaction has a second linear polymer reacting with the active sites of the chains in the first. The conversion rate is high, the steric demand is high, there are no termination reactions, side reactions are low, and the reaction time is dependent on the MW desired. The steric demands of the two polymers means that graft density tends to be low. This can be improve by long reaction times. Because the starting polymers have already been purified, however, it is possible to obtain polymers with low polydispersity indices. This strategy requires a telechelic polymer.
Section 5
Dendrimers
Dendrimers are unique in that they are perfectly 3-dimensionally branched polymers. They represent an entirely new class of polymers with properties and applications their own. They have monodisperse molecular weight, and are the first polymers to have such. The viscosity of dendrimers, once past the 'starburst' limit, begins to decrease with increasing molecular weight.
The synthesis of dendrimers is bio-inspired. They have a 'snowflake-like' structure: much more rounded and space filling, rather than linear. They are synthesised in a stepwise fashion, and can require protection/deprotection chemistry, much like solid-phase protein synthesis. Most of the linear polymers we've encountered have been synthesised as dendrimers. Dendrimers are synthesised either divergently or convergently.
Divergent synthesis has a centre, eg. ethylene diamine. This undergoes exhaustive (as many as is possible) 1,4 conjugate addition, so it now has four ester 'tails' spread out around it. These 'tails' all have a core molecule added onto them, and so the process repeats. The early steps have the reagents in large excess, to ensure that the reaction has gone to completion. This goes on for between 5 and 10 'generations', each of which consists of one layer of amine, and one of ester. The limit is called the 'starburst limit'. These dendrimers thus have a 'globular' shape, with large cavities and minimal chain entanglements. They are also monodisperse, which make for lots of interest. This method of synthesis is faster, cheaper and less laborious.
In theory hyperbranched polymers should have many of the same properties as dendrimers. The main difference is that some of the chains do not have branching, whereas in a dendrimer every position has branching. This can be a drawback for drug synthesis, for example, but for materials it may not be so important. Hyperbranched polymers can be produced in a single step. Hyperbranched polymers also show the increasing molecular weight-decreasing viscosity phenomenon that dendrimers show, but there is as yet no explanation. The more branching there is, the more they exhibit this effect.
Convergent synthesis has some advantages over divergent synthesis. It offers control of the location of surface functional groups. Chemically different dendrons produced by this method can be combined into one dendrimer. The intermediates are more easily purified, and the core is more easily replaced, meaning that there is more chemical flexibility.
This method starts from what will be the surface, and works inwards. Once the outer segments have been constructed, they are all attached to the core. There is no rule that all segments must be the same, giving greater chemical flexibility. If the wrong core is chosen, either it will not react or the dendrimer will be floppy, with an ill-defined 3D shape. In addition, there is no solid starburst limit, so it is harder to determine the optimum size and shape of the molecule.