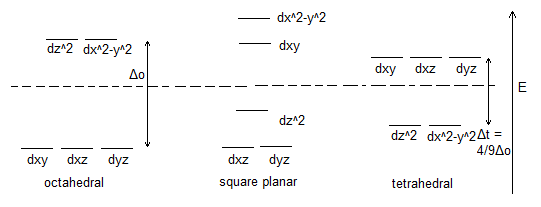Mesyltoe 3I3
Advanced Transition Metal Chemistry
This is a summary of a lecture course delivered in Autumn 2012 by Drs Silvia Diez-Gonzalez and James Wilton-Ely.
Executive Summary
Alkenes, Dienes, Cyclopentadienyls, Arenes
- Dienes tend to bond in a cis-formation.
- The MOs of dienes are more complex than those in an alkene. Varying backbonding causes differing bond lengths.
- Cp is a multipurpose ligand that can be monohaptic, trihaptic or pentahaptic, allowing it to be a useful and adaptable ligand.
- Cp can be tuned to very specific reactivity, making it very useful for catalyst design.
- The structure can be determined using knowledge of Fluxionality and NMR spectrometry.
- We describe polymers and tacticity in terms of diads and triads. A meso diad is one where both substituents point in the same way. A racemo diad is one where each point in opposite directions. A triad can be each of these things and heterotactic, AAB.
- Arenes bond in much the same way as Cp.
Carbenes, Carbynes, Alkyls, Aryls, C-H Activation
Alkenes, Dienes, Cyclopentadienyls, Arenes, by JWE
Revision
Revision: Crystal Field Theory
Crystal field theory predicts, in cases of a positive metal surrounded by negative ligands, which of the metal's d orbitals will rise in energy and which will fall in energy, separate for each geometry. This corresponds well with experimental findings.
Revision: The 18 Electron Rule
The 18 electron rule for octahedral complexes states that it is (generally) most favourable for complexes to have 18 electrons. This is because in the MO diagram for these complexes, there are 6 bonding orbitals and 3 non-bonding orbitals. Any more than 18 electrons, and they must be located in anti-bonding orbitals. However, sometimes filling the non-bonding orbitals is unfavourable, meaning that complexes have between 12 and 18 electrons, and these configurations can also be stable. The MO diagram for the octahedral complex also shows us the origin of the Δoct above. This is the energy gap between the non-bonding and anti-bonding orbitals.
For electron counting, metals are always considered as neutral. They are assigned the elemental number of valence electrons (including both s and d electrons). Ligands are classified as either L (donating 2 electrons), X (donating 1 electron) or Z (donating 0 electrons. Z are the rarest by far. For a neutral complex, the oxidation state of the metal is the same as the number of X ligands. Metal metal bonds count for one on each centre. Bridging X ligands count for 1 on one centre and two on the other, and bridging L ligands count for one on each.
Revision: Ligands and Terminology
η denotes hapticity, telling us how many ligand atoms are bonded to the metal centre (such as the η5 cp ligand), whereas μ denotes how many metal centres are bonded to the ligand (for example in bridging ligands).
Carbonyl ligands bond by sigma-donation and pi-backdonation. The reactivity of a carbonyl ligand depends on the degree of backbonding. This is because when the metal centre donates electron density back onto the carbonyl ligand, it donates into an anti-bonding orbital. The more backbonding there is, the stronger the metal-C bond, but the weaker the C-O bond. Electron poor metals, obviously, don't have enough density to backbond a lot. In these cases, the C=O ligand acts as a π acceptor. The frequency of the C=O bond in an IR spectrum is high, as the bond is strong. Electron rich metals have more electron density, so can push it onto the carbon, making the C=O a good π donor. The C=O IR frequency is low, as the bond is weaker.
Alkenes bond via π orbitals, donating electron density into empty metal d-orbitals. Because the alkenes are weak bases, this needs to be stabilised by another bonding contribution, for example, back donation from the metal into the alkene anti-bonding orbital (owing to symmetry contraints). The bonding of metals to alkenes is quite similar to the bonding of metals to carbonyls. As in the carbonyl example, backbonding weakens the C=C bond, meaning its IR frequency is lower the more backbonding there is. It also changes the geometry of the carbons, making them more tetrahedral and less trigonal planar. The backbonding is dependent on the energy of the complexes frontier orbitals (is it favourable for back-donation to occur?), steric effects (is there the necessary overlap?) and the Lewis basicity of the alkene (are there electron withdrawing substituents?). When there's loads of backbonding, and the C=C bond length becomes more like a single bond length, a metallocyclopropane is formed. Co-ordinated alkenes with no backbonding are susceptible to nucleophilic attack, but the more backbonding there is, the less susceptible to nucleophiles the ligand is.
We can't use this example to apply to all alkenes. When a system is conjugated (butadiene) it becomes more complicated.
Complexes
Complexes of Conjugated Dienes
Dienes have lower lying π* orbitals, making them better π acceptors than mono-alkenes, described above. They often have C-C bonds of equal length when co-ordinated, and tend to bond in an s-cis conformation (where s stands for single), almost as though they were a fragment of a ring.
They can be made by displacing other L-type ligands (such as C=O), and then heating to ensure high hapticity. They can also be made by displacing carbonyls with alkyl-halides and then eliminating the halides. They can be added to electron deficient precursors by removing ligands before adding the diene. A less common method is metal-vapour co-condensation, where metal and diene are mixed in the gaseous phase.
Free butadienes are usually very reactive, but they are notably unreactive when co-ordinated. They show no Diels-Alder reactivity and are difficult to hydrogenate, meaning that metal complexes (such as Fe(CO)3) can be used as protecting groups for dienes in larger molecules. This is because there are so many bonding orbital combinations that the M-C bonds are very strong.
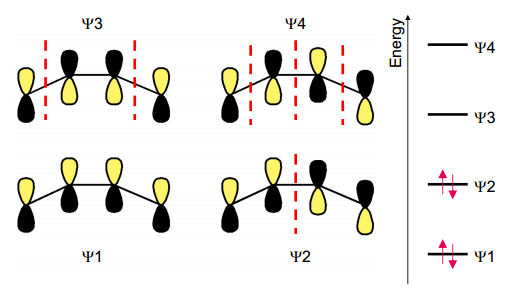
Although these complexes usually have C-C bonds of equal length, depending on the structure of the complex there can be some small variation. This is because of the node positions in the anti-bonding orbital, ψ3 (although it is possible in theory for backdonation into ψ4 to occur, it has never been observed).
From the MOs given above, we can see that if backdonation is occurring into ψ3, the central C-C distance will contract, whilst the end C-Cs with lengthen and weaken. If ψ2 is the HOMO, the central C-C distance will lengthen, and the end C-Cs will shorten. If some backbonding occurs, but not a great deal, we will see C-C bonds of equal length.
Metallocenes
Cp (cyclopentadiene) is the most studied ligand in organometallics. The Cp ligand is anionic, so complexes are usually made by transmetallation with Na or K. The metals are simply added to the Cp, and the Na-Cp adduct reacted with a metal chloride. The reaction is driven by salt formation. It is also possible to react Cp directly with a metal salt, but if the metal salt is insufficiently basic an auxilliary base is required (such as Et2NH). Sometimes a reducing agent is also needed.
The manner in which Cp bonds to the metal centre is dependent on where the metal is in the periodic table. Alkali, heavy alkali-earth and lanthanides bond ionically, with the number of Cp corresponding to the ionic charge of the metal. Lighter alkali-earth metals, group 3 and those from group 12 (Zn, Cd, Hg) bond intermediately, with the heavy group 3s having a co-ordination number of 1, and the others co-ordination numbers of two. Most of the transition metals bond covalently in a molecular lattice, with two or more Cp rings.
The MOs of Ferrocene (for example) are large and complex, but we can get a very similar result using CFT. If we imagine the Cp ligands as charged discs approaching on the z axis, we can get a splitting pattern that corresponds with the MO picture quite well: the xz and yz degenerate, the z2 underneath, and the x2-y2 and xy degenerate underneath that. The z2 is non-bonding, and the other two pairs are antibonding and bonding respectively. This misrepresents the bonding in transition metallocenes, as they are covalent, but this model usefully predicts several properties, such as the M-C bond length (longer the more electrons are in the antibonding orbitals). Ferrocene has two MOs that bond the centre to the ring: one of the pz of Fe with the two ring π orbitals in corresponding phases, and another the same, but with the s orbital of iron. The z2 d-orbital cannot bond in this way because the ring's orbitals lie in the nodal cone.
Titanocene as a monomer doesn't exist: it reacts with C-H bonds to give dimers with bridging hydrogens between Ti, and bonds between two of the rings. Cp*2Ti does exist monomerically, as a paramagnetic, yellow 14VE complex, stable at 0oC. The steric hindrance provided by the methyl groups prevents it dimerising, but it can rearrange to replace a methyl with a methylene and add a hydride on the Ti centre.
Vanadocene is highly sensitive, 15 electron, but isolable. It's reactions correspond with its strong electron deficiency.
Manganese (II) is a high spin d5 ion, and has no CF stabilisation energy. It acts as a hard ion, so there is less covalent bonding character. It polymerises at low temperature, but above 158oC it becomes a pink monomer. It is high spin at rtp, but the energy difference is only 2kJmol-1. The Cp* analogue is low spin, as Cp* has a higher field strength. This form doesn't polymerise.
Reactivity and Uses of Ferrocene
Cp is usually a spectator ligand, and is inert, but in metallocenes it shows aromatic behaviour, meaning it can partake in the expected reactions, eg. a Friedel-Crafts Acylation. In this particular reaction, ferrocene reacts 3,000,000 times faster than benzene.
Ferrocene can be lithiated, and also undergoes other metallations, as the protons on the ring are rendered acidic by co-ordination to the metal centre. This opens the synthetic door to a lot of syntheses. This can be used as a means of tuning the reactivity of the metal centre. Cp*, for example, with its additional electron density, makes oxidising the metal centre much easier than Cp does. Cp* is also more kinetically stabilising, as it is larger.
Ferrocene's very specific redox potential means that it was used in sensors for blood glucose determination (through a series of three redox cycles). Polymers of ferrocene (whether ferrocene alone, or with silyl bridges) have big potential for organic electronics. They're also super useful as very specific catalysts, again, to do with the specific redox potential.
Bent Metallocenes
When other ligands co-ordinate to the metal centre of a metallocene, a bent geometry is enforced between the rings (tetrahedral about the metal centre). In these complexes, the Cp ligands act as protecting groups. These complexes only occur in metals with a low number of d-electrons, owing to the 18 electron rule. When the symmetry is broken, and the Cp ligands bent together, empty orbital lobes are exposed allowing extra bonding. The more sterically hindered the Cp rings, the less 'bent' the structure is able to become. Calculations suggest there are three of these lobes, with the occupancy depending on the d configuration of the metal. These orbitals usually act as acceptors, but can sometimes act as donors for Z type ligands (such as BF3).
Metallocene dihalides are classic examples of this class of complex, and they provide useful starting materials for a wide range of syntheses (methylation, hydrolysis, carbonylation). Metallocene hydrides are formed mainly by metals in the first two rows. They can be lithiated, halogenated, etc. Cp2ReH has two non-bonding electron pairs and is a base comparable to an amine in strength.
Theory and Applications
Why is Cp So Great?
Cp is a ligand that is very versatile, and can adapt to the 'needs' of the metal. It can be pentahaptic, trihaptic or monohaptic, donating the corresponding number of electrons, and can ring slip during a catalytic cycle as is necessary. In its pentahaptic form it can occupy many co-ordination sites on a metal, blocking them from reacting.
Fluxionality: What is it and what does it do?
Fluxionality is the rapid interconversion of different configurations of a molecule, all of which are equal in energy. The interconversion is thermally driven. Pentahapto Cp rings rapidly rotate around the z axis, giving a sharp singlet in NMR spectra (as the rapid rotation renders all protons equivalent to one another). This isn't the only kind of fluxionality possible, though. There can be ring slippage, η5 to η1 interchange, and η1 to σ bond shifts.
The rate of rearrangement is relatively slow, in the region of NMR spectra (10-1 - 10-9 seconds). Microwave and vibrational spectra are too fast to register rearrangements.
| Spectra | Timescale (s) | Energy barriers (kJmol-1 |
|---|---|---|
| Vibrational | 10-13 - 10-14 | c. 20 |
| Microwave | 10-10 | 0-20 |
| NMR | 10-1 - 10-9 | 35-100 |
So NMR is particularly good for 'seeing' slow, high energy motions in molecules. At low temperatures (down to -150oC) the motion is very slow, and the signals due to different species appear separate (a static spectrum). At high temperatures (up to 150oC) the signals coalesce to a single line in the mean position. In between these limits, line broadening and coalescing is observed. Fluxional systems can be useful to establish a definite structure (using a static spectrum) and to study the pathway of a rearrangement (for example, by measuring a spectrum at multiple temperatures, it is possible to tell what sort of arrangement is occurring). Metal carbonyl compounds have fluxional interchange between bridging and terminal carbons (called carbonyl scrambling).
Alkene Polymerisation
Group IV metallocene dihalides with aluminium alkyls as activators make excellent catalysts for the polymerisation of alkenes. The active species is a positively charged complex with one unoccupied co-ordination site:
The facile modification of the Cp ligands allows specific design to be used to produce either isotactic or syndiotactic polymers. For production of HDPE (high density polyethylene) a zirconocene (or Ziegler-Natta) catalyst is used with an MAO co-catalyst to abstract methyl and to methylate. HDPE consists of long chains, and is flexible, relatively strong and cheap.
The positively charged complex on the right is the catalytically active species.
In order to make LLDPE (linear low density polyethylene, which consists of long chains with single chain branching), a constrained geometry catalyst is used. This consists of a 'half-sandwich' Ti complex. A Cp ligand is bonded to the Ti, but there is also a dentate substituents on the ring, such as an amide. These catalysts are what allow fine-tuning to syndio or isotactic polymers.
Polymers
How we describe tacticity
As mentioned above, isotactic means that all substituents are 'pointing' the same way, and syndiotactic means they are alternating directions. We can talk about this in more precise terms by using diads and triads. These are two or three adjacent repeat units in a polymer. In a meso diad, the two units are orientated the same way, and in a racemo diad the two are orientated in opposite directions. A triad can be meso (mm), where all three point in the same direction, racemo (rr) where the units have the orientation ABA, and heterotactic, where the units have orientation AAB (mr). The mass fraction of isotactic triads is used as a measure of tacticity. We can also talk about tetrads and pentads. Each kind of 'ad is described by n-1 letters, as the letters describe the relationship between the substituents, not the substituents themselves.
Different Catalysts for Different Tacticities
For isotactic polymers, C2 ansa-metallocenes are generally used, whereas for syndiotactic polymers, Cs ansa-metallocenes are used. Why? Maybe I'll understand later. The crucial thing for having a high level of control over geometry is to link the rings, and to have a high degree of steric hindrance. If this is not the case, there is no control, and an atactic polymer results. Studying the way these catalysts work allows us to intelligently design our catalysts for better selectivity.
Metal Arene Complexes
Properties and Synthesis
Arenes have a rich co-ordination chemistry. They are generally hexahaptic, but can adopt lower co-ordinations leading to some of their reactivity (including dihaptic and tetrahaptic). They can be synthesised using metal halides, a halide abstractor, the desired arene and a mild reductant (such as aluminium powder). Compounds of many of the early transition metals may be made by this method. Another route is the atom vapour condensation method, the co-condensation of vapourised metal and ligand into a solution of low vapour pressure on a cooled surface. This technique is the only one for many simply bis(arene) sandwich complexes. Where more than one co-ordination site is possible, the metal co-ordinates to the ring with the highest index of local aromaticity (ILA), which is the terminal ring.
Bonding
The bonding in arene complexes is superficially similar to that in a Cp ligand. The splitting between the bonding and antibonding orbitals is smaller in the arene complex, because both the central atom and the ligands are neutral. The higher the charge of the central atom, the greater the splitting. In addition, δ backbonding is more significant in the arene complexes. Bis(arene) complexes also show magnetic behaviour similar to that of metallocenes.
Reactivity
Neutral arene complexes are air sensitive, though the rings may be stabilised by the addition of electron withdrawing groups. Cr(η6-C6H5Cl)2 is stable in air. Unstabilised, the complexes oxidise. Cr(η6-C6H5Cl)2 is kinetically inert, ligand exchange occurring only in the presence of a Lewis acid, though bis(arene) complexes synthesised by co-condensation are labile. Bis(arene) complexes don't undergo electrophilic aromatic substitution, though mono-arenes do. Metallation can be accomplished using nBuLi, and the rings in the complexes are more readily metallated than benzene alone. Ligand exchange can be used to synthesise mixed arene compounds, but mixtures often result.
One of the reasons why these reactions are possible is the complexes' ability to undergo ring slippage. The rings can spontaneously adopt η4 configurations in order to fulfil the 18 electron rule.
Half-sandwich compounds (containing only one aryl substituent) are more common. They can be produced by a variety of ligand exchanges, with cyano ligands, other aryl ligands, and carbonyl ligands. They can also be synthesised by addition of the arene to a chloro-metal complex with a halogen abstractor present. The metal centre withdraws electron density from the ring, increasing the acidity of protons on the ring, and also increasing the rings' susceptibility to nucleophilic attack.
Alkyls, Aryls, C-H Activation, Carbenes and Carbynes
Alkyls, Aryls and C-H Activation
Metal-alkyl and Metal-aryl Complexes
These complexes can be synthesised by nucleophilic attack, using an 'R-' reagent, or transmetallation using Grignard reagents, or Organolithium reagents and metal chlorides. There is also electrophilic synthesis using 'R+' reagents, using alkyl halides and metal carbonyl complexes. Oxidative addition is another method, but this uses only unsaturated low-valent complexes, and alkyl halides again. Alkenes can also insert into a metal hydride bond. These reactions occur as an equilibrium with a very low concentration of the product, but they're important for catalytic transformation of alkenes (such as hydrogenation, hydroformylation, etc.).
Alkenes insert far more readily into M-H bonds than M-R bonds (which is the opposite way around to C=O). Alkene polymerisations are exceptions to this trend, owing to kinetic factors. Alkenes that have electronegative substituents or steric strain can reverse this trend.
Decomposition pathways
M-C bonds are strong, so are very thermodynamically stable. Decomposition relies on the ease of the decomposition pathways available, meaning that the complexes are kinetically unstable. The major pathway is β-hydride elimination, when a hydrogen on the carbon β to the metal is abstracted by the metal, turning the alkyl into a dihapto alkene. This then readily dissociates. For this pathway to be used, there must be a vacant site on the metal to allow co-ordination of the hydride, an available β-hydride, and a M-C-C-H co-planar arrangement must be possible. This means that smaller metallacycles are more stable, as there is less possibility for the motion necessary to create planarity. It also means that double bonds to bridgehead carbons are unfavourable.
Another decomposition pathway available is α-hydrogen elimination. This is mostly observed when a β-hydrogen is unavailable, but Mo and Ta are prone to this pathway in any case. Similar to the above pathway, there must be a vacant co-ordination site for this to occur. The result is an intermediate of alkylidene structure (but it is not a final product!)
Reductive elimination is a possibility, if an alkyl and a hydrogen or two alkyl groups are cis to one another. It is less favourable than for acyl and aryl groups, but in square planar complexes, particularly, it can be particularly facile.
Aryl-Metal Complexes
Due to π-bonding possibilities, transition metal-aryl bonds are more stable than transition metal-alkyl bonds, but only if the alkyl group has β-hydrides. The preparation of metal-aryl complexes is very similar to the preparation of metal-alkyl complexes. The nucleophilic, or transmetallation route is the most popular approach. Oxidative addition is also often employed. Phenyl groups can bridge two metals, much as alkyl groups can in main group metals.
The most common decomposition route for biaryl complexes is reductive elimination, giving a biaryl product, which can remain co-ordinated to the metal centre. Oxidative addition and reductive elimination are the base of cross-coupling reactions. Less common decomposition products are metal-benzyne complexes, which can create metallacyclopropenes or metal-alkyne bonds with the benzene.
Fluorinated ligands have enhanced stability. The reason for this is not understood, but there are two suggested explanations. One is that the high positive charge on the metal causes the d-orbitals to contract, shortening the M-C bond and increasing the M-C overlap. The other explanation is that the low-lying π* orbitals increase backbonding, so increasing the M-C bond strength.
Ortho-substituted ligands can also confer extra stability on a complex. The bulkier the substituent, the greater the kinetic stability of the complex. Square planar complexes are particularly vulnerable to z-axis attack. Ortho substituted benzene as a ligand can prevent this line of attack. This doesn't prevent metallation of a hydrogen, however.
Agostic Hydrogens
If a metal is electron deficient, it can gain some electron density from C-H bonds of an alkyl ligand, creating a 3-centre 2-electron bond. The presence of these interactions is often suspected, but proof is hard to obtain. Neutron diffraction can be used to detect the deviation from ideal geometry caused by agostic interactions. 1H NMR shows that agostic hydrogens are shifted upfield, but the effect is often unclear, and solid NMR is required for it to be really seen. The coupling constants in NMR are smaller than usual, and the C-H bond displays lower wave number stretching frequencies.
This interaction can be used for C-H activation. Activation is the process of a transition metal facilitating the breakage of a (usually inert) bond. Functionalising alkanes with transition metals via C-H activation is a greener alternative to the use of many halides and other leaving groups. Specificity is the problem, as a molecule can have many C-H bonds. Also, oxidative addition of C-H to metals is thermodynamically unfavourable. This means that inter-molecular C-H activation is the most common. Activation can give a useful synthesis for metal-aryl complexes (by hydride co-ordinating to metal centre). The same is possible for alkanes, but far more challenging. An example is the selective borylation (with pinacolborane) of octane using a rhodium catalyst.
Carbenes and Carbynes
Carbenes
Carbenes are compounds possessing a neutral divalent carbon with six valence electrons. They can exist as singlet or triplet carbenes: these are called spin isomers. They can be in either a 'bent' structure (sp2 hybridised, with a lone pair occupying one lobe) or a linear structure (sp hybridised). The bent structure can be either a singlet or a triplet state, and the linear structure is always in a triplet state as the electrons singly occupy the non-hybridised p-orbitals. From an organic perspective, carbenes are thermodynamically and kinetically unstable, but in practice they are isolable with stabilising influences.
In inorganic chemistry, they (mostly) manifest as M=C bonds. In order for these bonds to form, they must be electronically stabilised and the geometry must make it possible for the metal and carbene orbitals to overlap (meaning the R-C-R angle must narrow). The metal-carbene bond is very thermodynamically stable, but it can be kinetically unstable in the right circumstances.
NHCs can be isolated as free species, so comparison of the ligand and compex can be useful for identification purposes.
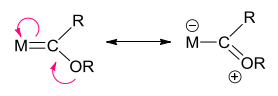
Fischer Carbenes
The actual bond order in Fischer carbenes is <2, due to the canonical forms possible:
As NHCs have two pi-donor ligands, they have even lower bond order. This explains the clear differentiation between carbenes that is possible using 13C NMR.
Fischer carbenes can be synthesised by the addition of a lithiated reagent to an M-CO complex, followed by a halogenated reagent (to remove the lithium). To synthesise from acetylide complexes (complexes containing an ethyne), add H+ to reduce the bond order, and then EtOH. There is also electrophilic abstraction from metal-alkyl complexes, where TMSCl is used to abstract F from a trifluoromethane ligand.
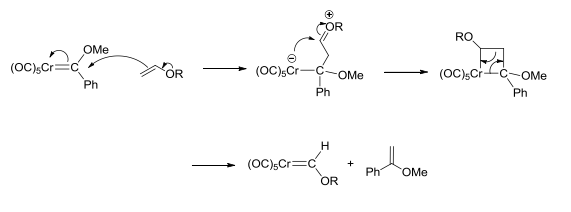
The electrophilic carbene carbon reacts with nucleophiles, such as amines, lithiated alkyls etc., to give Fischer carbenes with two alkyl substituents, which are particularly reactive. They can also be reacted with oxidants (such as cerium(IV) salts, pyridine N-oxide or air) to yield carbonyl complexes. This reaction can be used to characterise the original carbene. The carbenes can also react with alkenes to give 1,1 disubstituted alkenes, via a metallacycle intermediate. The mechanism of this is given below:
Shrock Alkylidenes
X-ray diffraction can be particularly useful for identification of Shrock alkylidenes.
Schrock alkylidenes can be synthesised by α-hydrogen elimination from a di-alkyl precursor. The eliminates an alkyl that has abstracted a hydrogen from the other alkyl ligand, which has now become a carbene. Deprotonation of a metal methyl complex (by a base such as NaOMe) gives an alkylidene, the presence of which can be confirmed by X-ray crystallography.
As a nucleophilic carbon, Schrock alkylidenes react with electrophiles. They form adducts with Lewis acids. They also react with alkenes, which is one of the most important applications of alkylidene complexes.
Alkylidene complexes can behave similarly to phosphorus yllids, to produce metal oxides and alkenes. They show strong agostic interactions, where other ligands interact with the H. This is because alkylidene complexes often have fewer than 18 electrons and the M-C bond is short.
N-heterocyclic Carbenes
NHCs are persistent carbenes: they are isolable and commercially available. The substituents on the nitrogens are tunable, as is the ring, which can be either saturated or unsaturated and substituted as is most useful. The Ns act both as π-donors and σ-acceptors to stabilise the carbene. There is a localized lone pair that provides the strong M-C bond.
Free N-heterocyclic carbenes can be prepared by deprotonating an imidazolium salt. To prepare the complexes from the free ligand, the ligand is metallated using CuX. They can also be made by transmetallation from another NHC complex.
The strong NHC-metal bond means that they frequently act as spectator ligands. However, they can react to degrade a complex. Reductive elimination can occur, with the active site of the NHC alkylating and forming a halide salt. There is also the possibility of a displacement reaction. Heating NHC complexes in dichloroethane with a replacement ligand (such as PPh3) can result in the NHCs dimerising with halide counterions.
Metal-Carbyne Complexes
Carbynes, complexes with a triple M-C bond, can be made from carbene-carbonyl complexes with BCl3, which removes the better leaving group substituent, and then replaces one of the carbonyl ligands with a chloride. The one that is displaced it the carbonyl trans to the carbene, as the trans effect is very pronounced. They can also be made by deprotonation of a carbene. Carbynes can exist either in a doublet state (one lone pair, one single electron) or in a quartet state (three single electrons). They are linear, with a very short M-C bond and 13C NMR in the 200-400ppm region.
Although there are two kinds of carbynes, the distinction is less marked than that between carbenes. There is the Fischer carbyne, which is a doublet, and similar to a Fischer carbene, has an electrophilic carbon. It bonds by σ donation and d→p π backbonding. The Schrock carbyne is a quartet, and bonds by one σ and 2 π bonds. The carbon is nucleophilic, and so reacts with electrophiles.
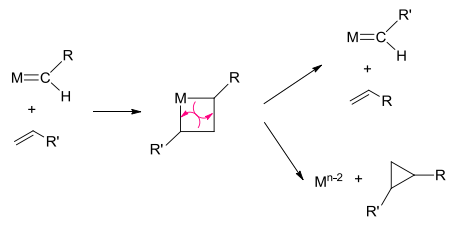
Metathesis Reactions
The Alkene (Olefin) Metathesis reactions are the exchange of CR2 units between two alkenes. The mechanism is very similar to a Wittig reaction. There are ill-defined general catalysts, used for simple alkenes, and well-defined catalysts, used in industry and academia for a wide range of unsaturated compounds.
For the reaction to proceed, the catalyst must be activated by either dissociative displacement (begun by dissociation of a ligand) or by associative displacement (begun by association of an alkene). Which method is preferred depends on the alkene and the nature of the ligands attached to the metal centre.
The Grubbs catalysts are Ru based complexes. It's relatively insensitive towards oxygen and moisture, and so easy to handle. It also is tolerant of many functionalities. The Schrock catalysts are Mo based. They have a higher activity than the Grubbs catalysts, but are very sensitive to air and water, so require delicate handling. They're tolerant towards S, P and CN functionalities, but not protic or aldehyde functionalities.
Ring closing metathesis is the application of this reaction class with the biggest impact in organic synthesis. This is the use of the above catalysts to join the two unsaturated ends of a molecule together intra-molecularly. Ethane is also released during the process, and this drives the reaction. For small and medium rings, the bonds formed are exclusively Z owing to steric strain. In larger cycles, this is no longer an issue, and so the cycles are formed without stereospecificity.
Cross metathesis is metathesis with the aim of creating difunctionalised molecules. It is difficult to prevent dimerisation across the double bonds, and also stereospecificity is challenging. This can be made easier by the use of allyl silanes for E selectivity. Ring opening metathesis polymerisation occurs when one of the alkene bonds to be broken is within a cyclic molecule. The greater the release of ring strain, the more favourable the position of equilibrium. Polymerisation using this method can also be performed on dienes.