Mesyltoe 3I2
3.I2 Advanced Main Group Chemistry
A summary of a lecture course given by Dr. Paul Lickiss in Autumn 2012
Highly Reactive Compounds
Radicals
There are three ways of making radicals: homolytic bond cleavage (by heat or light, particularly of halogen or peroxide bonds), oxidation of anions or reduction of cations. Radicals can abstract hydrogens (eg. from solvent), dimerise (which often has no energetic barrier) and disproportionate, one radical becoming anionic, and one cationic (or near enough). They're implicated in polymerisation (free radical polymerisation) and the destruction of the ozone layer! They're also important in noble gas chemistry.
Radicals can be stabilised either electronically (delocalisation of the radical charge) or sterically (surrounding the reactive centre with steric bulk to prevent reaction).
Group 14 radicals
Carbon radicals can be planar, flexible pyramidal (allowing easy inversion about the carbon) or rigid pyramidal, and go in that order in terms of stability. In contrast, Si radicals are more stable as pyramidal structures. This is because the smaller electronegativity of Si results in higher energy MOs, meaning that orbital mixing (or hybridisation) is more favourable. We can tell this using EPR spectroscopy. Higher hyperfine coupling is observed with higher orbital s character, so we can tell that the SOMO has a large quantity of s character if it has large coupling. This shows that for radicals in solution, Si is the only group 14 element that prefers a pyramidal structure.
Dimer radicals
Diborane radical anions are examples of radicals stabilised by sterics. When they have mesityl substituents, the repulsion between these bulky substituents keeps the solid-phase bond legnth constant. In dialane radical anions, this steric repulsion can be sufficient to break the bond. This can also occur with phosphorus compounds.
Interhalogens, noble gas compounds and fluorocarbons
Radical chemistry is used in the production of CFCs. Light activated chlorine is reacted with methane, giving tetrachloromethane. This is then fluorinated (using HF and SbFCl4 catalyst) and processed further to give CFCs, which as we all know, destroy the ozone layer. This occurs by radical chlorine abstracting oxygen from ozone and trapping it in water and nitrogen dioxide. But CFCs aren't all bad! Chloro-difluoromethane is thermolysed to give tetrafluoroethylene, which when polymerised gives Teflon (PTFE). Hurrah!
Interhalogen compounds are surprising, but exist. Diatomic compounds aren't so surprising, but it's possible to have heptavalent iodine (in IF7). Many of these compounds are powerful fluorinating agents. One particularly powerful is ClF3, which is a better fluorinating agent than fluorine itself. Most of these compounds are prepared from the elements in an appropriate ratio under varying conditions. The bond strengths in these compounds reflect the electronegativity difference between the compounds, so IF is the strongest bond, and BrCl the weakest. As we might expect from this, the weaker the bond, the higher the temperature required for the reaction to occur.
The structure of the complexes conforms to VSEPR. The bonding depends upon the geometry of the complex. In complexes of trigonal bipyramidal overall geometry (regardless of whether vertices are occupied by electron pairs or not) the axial bonds are 3 centre, 4 electron bonds. The equatorial, sp2 hybridised, orbitals hold bonding or lone pair electrons. In octahedral complexes, with fewer than six substituents, the atoms in the equatorial plane are bonded by 3 centre 4 electron interactions. The axial orbitals are sp hybridised, and these orbitals hold either bond or lone pairs. In octahedral complexes with six substituents, all are bonded by 3 centre 4 electron bonds.
As we can see from the MO diagram, this means that along the x axis there are two bonding electrons and two non bonding electrons. This is the origin of the 3 centre 4 electron bond.
Anionic complexes are much more common than cationic complexes, owing to the high electron affinity of the halogens. I3-, I5-, I7- and I9- are all known, and form bi-linear structures (two lines with an angle in between). They are stabilised by large soft cations, like R4N+. The cations are easier to form as the group is descended, and polyatomic iodide cations are the most common, which is as we would expect given this is the least electronegative of the halogens. Strong oxidising agents are required to form these. I5+ is linear with ends 'bent' in opposite directions. I3+ has a bent shape, like water.
Noble Gas Chemistry
Xe and O have a similar ionisation potential, so if it's possible to create oxygen based ionic compounds, then it should be possible to create Xe based ionic compounds. Turns out, it is! XeF2 exists and is commercially available, though it is easily hydrolysed to Xe, O2 and HF. KrF2 also exists, but is unstable at rtp.
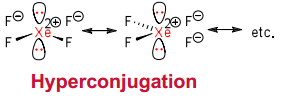
Obviously, since noble gases already have a full valence orbital, their compounds will be hypervalent. They really bond by a process of hyperconjugation, so:
This means that the average Xe-F bond order is 0.5.
Xenon fluorides react with lewis acid species that can act as fluoride acceptors, to give XeFnMF5 (or similar) products. XeF6 in particular attacks glass, yielding silicon fluoride (the formation of which is the driving force) and XeOF4. Xenon fluorides when hydrolysed produce XeO3, which is highly explosive, and HF. At high pH, XeO3 becomes hydroxenonicacid (?), HXeO4-. XeF2 oxidises and fluorinates simultaneously. It is more reactive in the presence of HF. The 3c, 4e bonds in XeF2 and XeF4 are strongly polarised, and positive at Xe, making Xe susceptible to nucleophilic attack by carbon species. Substitution reactions at Xe are known, but the products tend to be thermally unstable.
Xe can act as a ligand in transition metal complexes, stabilised by weakly co-ordinating anions such as Sb2F10-. KrF2 noted above can act as a strong fluorinating agent for both Xe and Au.
Heavier Carbene Analogues
Carbenes
In a carbene, the theoretical triplet state (which is the ground state) consists of two singly occupied degenerate p-orbitals, perpendicular to the linear bonding plane. In reality, the bonding is bent, and the carbon is sp2 hybridised. One of the electrons sits in one of these orbitals, whilst the other sits in a p orbital. This means that the two electrons are not degenerate, but the energy separation is smaller than the pairing energy. The bent tripet has diradical reactivity.
The triplet state of a carbene can be stabilised by enforcing linearity, by adding sterically bulky groups, delocalising the spins and by preventing addition at the para positions by using up all the carbon's valence electrons.
Silylene
The silylene ground state is a singlet. It is more bent than the carbene. The lone pair has more s character, and the orbitals approach an unhybridised state as the pz orbital is empty. It is both electrophilic and nucleophilic. This means it has a lot of varied rectivity, making it very industrially useful. The singlet state is so stable for SiH2 for the same reason that the Si radicals are pyramidal: the bending and orbital mixing is more favourable due to Si's lower electronegativity. The mixing would not be possible in a linear situation: it is the breaking of symmetry via bending that allows the orbitals to be of the same symmetry, and thus to mix.
Carbene analogues in the other main groups include phosphines and borylenes. Compounds similar to NHCs can be made with selenium.
Stabilisation and reactivity
NH substituents can stabilise all carbenes and carbene analogues (silylene, germylene, etc.) by aromatic stabilisation. These have little effect on reactivity, but a profound effect on stabilisation.
Heavier Multiple Bonding
Bonding in Main Group Elements
Double bonds have less stability the lower down the groups you go. This is because the orbitals are more diffuse, so there is less overlap of electron density, so the bonds are longer. The bond energy includes the energy required to produce a triplet, that is, the gap between a singlet and a triplet state.
The Charles-Goddard-Malrieux-Trinquier model has two different models of double bonding. Where the bond is made up of two singlet fragments, there is a high preparation energy, and this is greater than the approximated π bond energy. This results in a trans-bent double bond, with two sp2 hybridised centres leaning together for overlap between the two p orbitals and two of the sp2 orbitals. There is a smaller HOMO-LUMO gap, and a weaker double bond. Where the bond is made of two triplet fragments, the preparation energy is less than the π bond energy. The double bond is more planar, and the centres interact as we traditionally think of bonds interacting. There can be very large substituents. Any structure distortion tends to be twisting rather than trans-bending.
These different structures affect the HOMO-LUMO gap. The bent structure (where one centre has its substituents 'up', and the other 'down') has a smaller HOMO-LUMO gap than the perfectly planar structure, but a larger HOMO-LUMO gap than the 'twisted' structure. In the twisted structure, where the two substituent planes are at right angles to one another, the HOMO and LUMO become degenerate, and they are both non-bonding, singly occupied MOs.
Disilenes are structurally flexible, and can take up any of these structures depending on circumstances, such as electron density in the solvent. The HOMO-LUMO gap affects the colour of these compounds.
Si=Si Reactivity
Disilenes are synthesised by elimination of halide salts: a dilithiated silicon with a dihalogenated silicon, or a dihalogenated silicon with a source of lithium. Oxygen can be added to Si=Si bonds facilely, owing to the low bond enthalpy. It adds to form a dioxygen, disilicon four-membered ring, which then rearranges so that the oxygens are bridging. The Si-Si distance is shorter than a normal bong length in this structure. To create epoxide analogues, N2O is used instead. A similar phenomenon happens with any addition reaction. Because the bond is so much weaker than its C=C equivalent, the additions are much simpler. The diffuse orbitals mean that four membered rings are possible, as there is less ring strain.
Like alkenes, disilenes can also coordinate to transition metals either mono or dihaptically. Dimetallasilanes can undergo transmetallation to the same centre to produce silane-metallacycles, or η2 co-ordinated disilanes.
Diplumbenes are also possible, and take up a trans bent structure.
Sila- and tristanna-allenes
Sila-allenes are arene-silicon analogues, like heteroaromatics without the additional electron density. The electropositive nature of silicon allows it to exist in these compounds with only two bonds, like a carbene. Tristanna-allene is a compound made up of a ring of three tin molecules, synthesised from distannenes.
Group 14
Potassium graphene can be used as a halogen abstractor in the synthesis of Si-Si triple bonds. A tribromosilane is reacted with potassium graphene. This removes 2 equivalents of bromine, and dimerises the silane. If this is repeated, another four equivalents of bromine will be eliminated, and a Si-Si triple bond results. Unlike alkynes, this has a bent geometry around either silicon. This can then be hydroborated without a catalyst, or aminated. Both methods give novel syntheses of disilenes. Bullky protecting groups are necessary for these compounds to prevent dimerisation.
Digermene and digermyne complexes also exist. It is the buildup of incipient lone pairs on Ge that gives the bending. Distannyne species have also been found. The distannyne has a reversible reaction with ethylene, forming a six membered ring with two tins and four carbons. This has a possible application in ethylene/ethane separation.
Just as the above compounds require bulky substituents for stabilisation, NHCs can also be used for stabilisation. In diborane, NHC stabilisation allows the molecule to be stable at room temperature. Diborane is made from trichloroborane, KC8 and the stabilising NHC.
Digallynes have not been isolated. The bond length of some compounds suggests a triple bond, but this is probably not the case. Complexed Na+ are sandwiched by aryl rings from the bulky substituents, artificially shortening the bond, as the sodium atoms are also complexed to the gallium atoms.
The existence of a ferrogallyne (triple bond between gallium and iron) has also been suggested, due to the short bond distance, but calculations suggest that there is no π overlap. The actual interaction is a dative covalent bond.
Alkyne analogues become increasingly bent as the group is descended due to the inert pair effect, where the electrons in the s orbital are two low in energy to take part in bonding, and so remain non-bonding. The lower down the group an atom is, the higher the likelihood of the lone pair effect.
Group 15
Diphosphenes are made by reacting a dichlorophosphene with Mg, causing to dimerise and eliminate magnesium chloride. The bulky groups for stabilisation are, again, necessary, and the structure is bent, owing to those pesky lone pairs. When reacted with an excess of Methyl Triflate, a disphosphenium cation results, with the triflate being the anion.
Dehydrohalogenation is used to make diarsenes. This is achieved using DBU, and involves, you've guessed it, the removal of hydrogen and halogen atoms, which complex with DBU.
A Bi=Bi compound has been made, starting from BiCl3, going via a Bi-Se 6 membered ring, and after addition of phosphine (to remove the Se), the Bi=Bi compound resulted. Like the Si=Si compound, this can be reacted with oxygen to give a compound with bridging oxygens.
Heteronuclear Double Bonds
Silenes and germenes are classes of compound that have a main group element double-bonded to carbon. This can be achieved by light activating a silyl-carbonyl compound, causing a 'Brook rearrangement', with one of the R groups from the Si migrating to the oxygen. It can also be achieved by eliminating a salt from a silyl-alkyl (having a halogen and a leaving group, one on C, one on Si/Ge). Heating these with BuLi lithiates the halogen position, and warming again removes the lithium with the other leaving group as a salt. The lithiated position is stabilised by the electropositivity of the α atom. These silenes and germenes can dimerise to form four-membered rings (usually 'head to tail'). ROH can be added to them to give a C-H bond and an ether bond. Oxygenation can cleave the bond to give a carbonyl and a Si=O, the latter of which can form six membered rings. Silenes can be coordinated to transition metals by β-hydride elimination or by reduction. Sila-aromatics are also possible.
Heteronuclear double bonds can be between carbon and phosphorus. A phosphene reacts with an acyl chloride, causing the loss of a TMSCl, and the formation of a single P-C bond. Another R group then migrates (as in the Brook rearrangement) to the O of the carbonyl. All the above reactivity also applies to these bonds.
Silanones are particularly difficult to prepare. Silyl alcohols are reacted with silanes, and the bonding is such that there is a Si-O-Si bond. This is then reacted with either N2O or CO2 to give a silanone.
Group 14 to Group 16 Double Bonds
These are more difficult to achieve, as only one end can be sterically protected. It isn't impossible though. Silicon diprotectinggroup dihalide can be lithiated, then reacted with sulfur to form the fifth member of a ring. Phosphine is then used to remove the sulfur (much as it does in the Wittig reaction) and Si=S is the result. Similar reactions are possible with germanium/tin and sulfur/selenium. Lead will react with sulfur in this way. These multiple bonds undergo Diels-Alder reactions readily, unlike carbon analogues.
'Triple' bonds
A phosphorus carbon triple bond has been observed, effected by elimination of a silyl ether. The possibility for triple bonding decreases as you descend group 14, due to the inert pair effect. Germanium and tin only double bond to themselves, and lead will only single bond.
Points to Remember
- Atoms get larger and bonds weaker on descending group
- Pi interactions get weaker down a group, and are weaker than sigma interactions.
- Oligomerisation maximises strong single bonds, and minimises weak multiple bonds.
- Steric protection is necessary for weak multiple bonds.
- Bulky groups like TMS3E or heavy substituted aryls are good steric protectors.
- Synthetic routes often involve photolysis or salt elim.
- Fewer substituents around a multiple bond means they must be larger.
- Geometry around multiple bonds is influenced by lone pair character.
A final soul-crushing point:
“The classical multiple bond indicators – bond lengths and bond strengths – have no meaning for multiple bonds in which elements from the higher periods are involved. However, they are valid for an exceptional element: carbon” Grutzmacher and Fassler, quotation from Chemical Communications, 2000, 2175-2181.
Anions
Recap: "Carbon Anions"
We make 'carbon anions' by metallating a haloalkane/ene/whatever, usually with lithium, but sometimes with magnesium (Grignard) or copper. The electropositivity of the metal allows the carbon to behave as though negatively charged. Organolithiums, in particular, cluster to stabilise. The degree of clustering depends on how electron-rich the solvent is. Grignard reagents undergo Schlenk equilibrium, where they dimerise (with bridging halides/carbons), exchange substituents, separate and so on.
Grignard Compounds
β-diketoiminate ligands (like a pyridine, but with two Ns and an H in between) can be metallated with lithium, then transmetallated to a Grignard. In the presence of potassium, Grignards dimerise, bonding through the Mg. X-ray crystallography and NMR have proved that there are no hydride bridges.
Instead of magnesium, calcium and other group 2 metals can be used. They are uncommon because the metals are relatively unreactive: CaO will not dissolve in THF or ether, so must be activated. This is done using 1,2-dibromoethane, to brominate, and then sodium, to debrominate and leave the activated Ca (called Reicke-calcium, pyrophoric). This is then reacted with an alkyl halide. Organocalcium compounds have high reactivity. They have a tendency to rearrange, and to cleave ethereal solvents.
Mixed counterions
Mixed counterions are when two different metals (for example, lithium and magnesium) are used to make a carbon 'anionic'. An example of this is the Schlosser base, LICKOR, produced by mixing n-BuLi with t-BuOK. Its basicity is between nBuLi and nBuK. It displays higher selectivity and 'suppresses erratic side reactions'. Another example is the so-called 'Turbo Grignard', TMP lithium mixed with MgCl2. This has enhanced reactivity and is regioselective. (TMP is tetramethylpiperidine).
Boryl anions
All other main group anions (give or take) satisfy the octet rule. Boryl anions, having only six electrons, do not. They can be synthesised from halo-diaminoborane, metallated, which gives a diaminoboryl anion. This anion can be stabilised using an NHC analog. Diisopropylethylenediamine is treated with magnesium. It is then reacted with BBr3, which is then lithiated, giving a five membered ring with an N-B-N series and a lithiated B. This can then be transmetallated. There is a negative charge on the boron, so it has higher nucleophilicity and no electrophilic reaction are reported.
Anionic E=E compounds
Disilenes can be lithiated and that chemistry taken advantage of.
Weakly co-ordinating anions & cations
Non-coordinating anions in the condensed phase do not exist, but a similar effect can be obtained by having many weak interactions rather than one strong reaction. This means there must be negative charge over a large area, to give spread out nucleophilicity, so there is no obvious attack point. Tetra(pentafluorophenyl)borate is an example of this.
Cryptands and crown ethers turn contact ion pairs (CIPs) into solvent-separates ion pairs (SSIPs), leaving a naked anionic centre, and increasing nucleophilicity. These become weakly co-ordinating cations.
Cations
Group 14 Cations
Olah's trimethylcarbenium ion, a positively charged t-butane (stabilised through hyperconjugation) is an example of a main group cation. It ought to be possible, given Si's electronegativity, to make a similar cation from silicon. Such ions are readily observed in mass spectra, but rarely in solutions and solids. Part of this is due to leaving group ability. Many of the leaving groups that work well with carbon do not work well with silicon, as Si-X and Si-O bonds are very strong (due to the very electropositivity that makes it so theoretically promising). This means solvolysis removing a leaving group is not an effective route. Solvolysis of perchlorates (such as Ph3SiOClO3) has been found to work, however, by solvent abstraction of the chlorate portion of the molecule.
The solvent used much have low nucleophilicity: nitriles, ketones, amide, ethers and amines all act as donors. Water and alcohols react to give silanols. Low polarity solvents, such as hexane, cannot dissolve ionic species. Toluene or chlorobenzene are the solvents of choice. The anion used must be very poorly coordinating and unreactive. Halogenated carboranes or tetra(pentafluorophenyl)borate both work. The substituents at Si must be large to protect the electrophilic centre from reaction. There must be no donor groups. The Si-H bond is weaker than the C-H bond, meaning that Ph3C+X- can abstract the hydrogen successfully. The resulting complex, with Si co-ordinated to the now positively charged solvent ring, is like a Wheland intermediate.
Heavier Group 14 Cations
The allyl route to group 14 cations can also be used on the heavier cations. Trimesitylsilylprop-3-ene is reacted with triethylphenylsilicon. The double bond of the propene opens to bond with the other silicon, leaving a positive charge in the chain. The Si-C bond breaks to recreate a double bond, and leave a Mes3Si+ cation. Tin can also be prepared in this way.
Reactions of very sterically hindered Si gives surprising results: intramolecular arrangement or intermolecular exchange can cause bridging SiMe2 groups.
Borocations
Borocations are classified by the number of neutral ligands bonded to the central atom. All three kinds are +1 in charge, but a borinium has no ligands, a borenium has one, and a boronium has two.
NHC analogues
Phosphorus can form NHC analogous cations as part of 5 membered rings with N-P-N cluster. The P atom carries positive charge, making it ambiphilic, unlike the Group 14 examples, which are nucleophilic. Sulfur has similar chemistry, as does selenium (though both of these carry a +2 charge). These can act as atom transfer reagents.