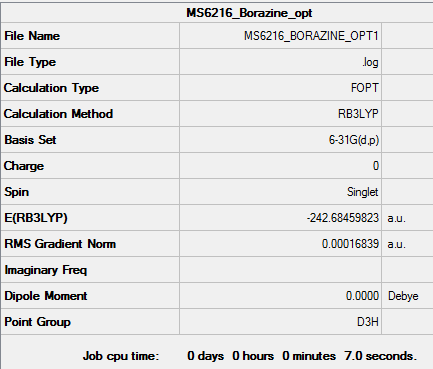
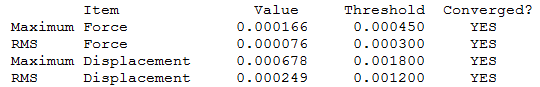
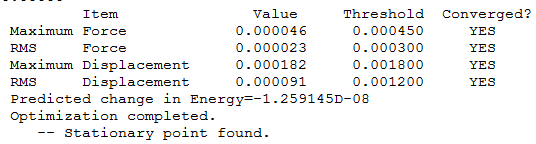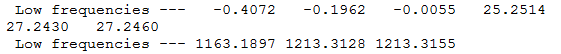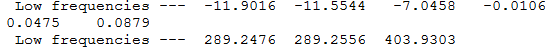MS6216 wikipage
BH3
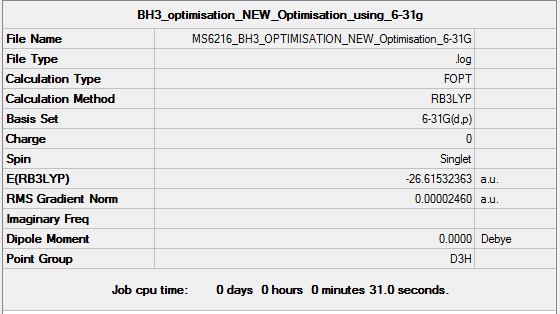
Method and Basis set used
Method : RB3LYP Basis set : 6-31G(d.p)
Summary Table
Item Table
Log File
File:MS6216 BH3 SYM OPT D3H SYMMETRY FREQUENCY.LOG
Low Frequency Values
BH3 |
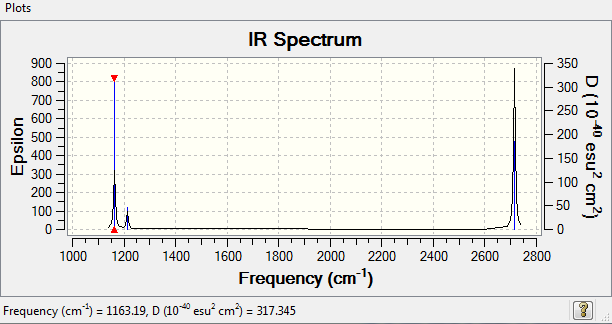
Vibrational spectrum for BH3
| wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1163 | 92 | A2 | yes | out-of-plane bend |
| 1213 | 14 | E' | slight | bend |
| 1213 | 14 | E' | slight | bend |
| 2582 | 4 | A1' | no | symmetric stretch |
| 2714 | 5 | E' | yes | asymmetric stretch |
| 2714 | 5 | E' | yes | asymmetric stretch |
IR Spectrum
6 peak aren't seen as some of the vibrations are degenerate (occur at the same frequency therefore overlap and are not visible.
Ng611 (talk) 16:32, 21 May 2018 (BST) What about the mode at 2582 cm-1?
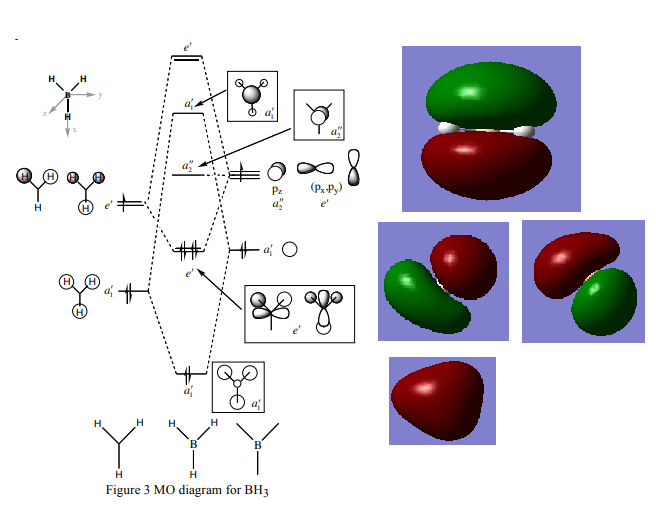
BH3 MO Diagram
MO diagram taken from: http://www.huntresearchgroup.org.uk/teaching/teaching_comp_lab_year2a/Tut_MO_diagram_BH3.pdf
Ng611 (talk) 16:35, 21 May 2018 (BST) Where are the computed MOs for the 1a' and 2e' orbitals?
The Linear combination of atomic orbitals approximation predicted the orbital shapes well however as seen in the pictures the orbitals do not remain separate and combine to form molecular orbitals showing some orbitals 'fused-like' as one orbital. There is a slight difference in interactions between electrons are not taken into account but this model still is accurate and useful.
Ng611 (talk) 16:35, 21 May 2018 (BST) The 'fused nature of the computed MO's is a cosmetic difference, and is not necessarily significant. Your description about 'interactions between electrons' is a little vague -- what do you mean by this? You are right to conclude that qualitative MO theory is accurate and useful, however.
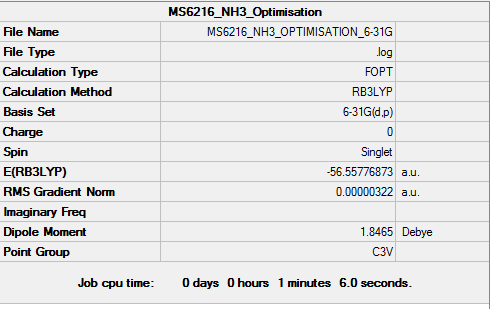
NH3
Method and Basis set used
Method : RB3LYP Basis set : 6-31G(d.p)
Summary Table
Item Table
Log File
File:MS6216 NH3 OPTIMISATION 6-31G FREQUENCYANALYSIS.LOG
Low Frequency Values
NH3 |
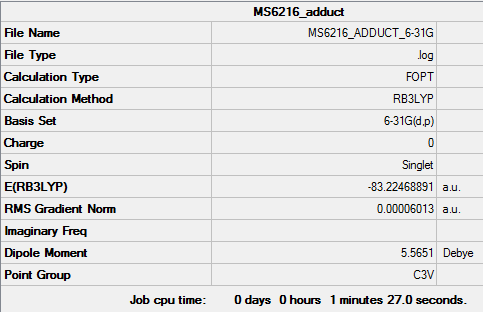
H3BNH3
Method and Basis set used
Method : RB3LYP Basis set : 6-31G(d.p)
Summary Table
Item Table
Log File
File:MS6216 ADDUCT 6-31G FREQUENCY.LOG
Low Frequency Values
H3BNH3 |
Adduct Energy
E(BH3)= -26.61532 a.u. E(NH3)= -56.55777 a.u. E(NH3BH3)= -83.22469
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)] = E(-83.22469)-[E(-56.55777)+E(-26.61532)] = -0.0516 a.u. = 135 kJ/mol
It is a weak bond as when compared to the C-H bond energy (338 kJ/mol) it is only roughly one third of the bond strength.
Ng611 (talk) 16:36, 21 May 2018 (BST) Remember to cite your bond values (ideally from a textbook, databook, or paper).
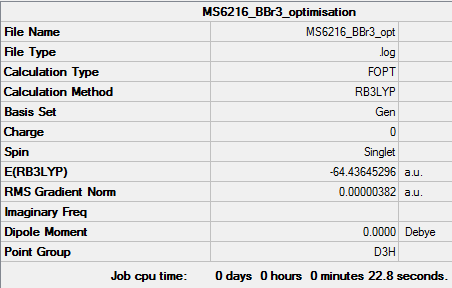
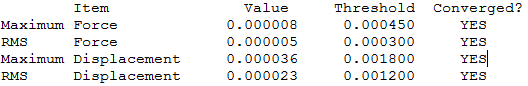
BBr3
Method and Basis set used
Method : RB3LYP Basis set : Gen Ng611 (talk) 16:37, 21 May 2018 (BST) You mean LANL2DZ, right? Gen is not a valid descriptor in job information.
Summary Table
Item Table
Log File
Low Frequency Values
DSpace
BBr3 |
Aromaticity
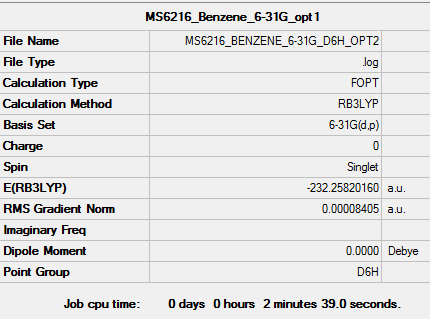
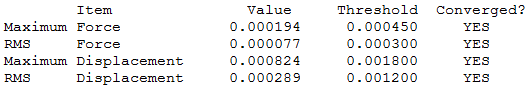
Benzene
Method and Basis set used
Method : RB3LYP Basis set : 6-31G(d.p)
Summary Table
Item Table
Log File
File:MS6216 BENZENE 6-31G D6H FREQ.LOG
Low Frequency Values
Benzene |
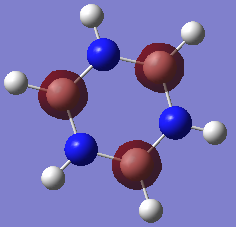
Borazine
Method and Basis set used
Method : RB3LYP Basis set : 6-31G(d.p)
Summary Table
Item Table
Log File
Low Frequency Values
Borazine |
Benzene Vs Borazine
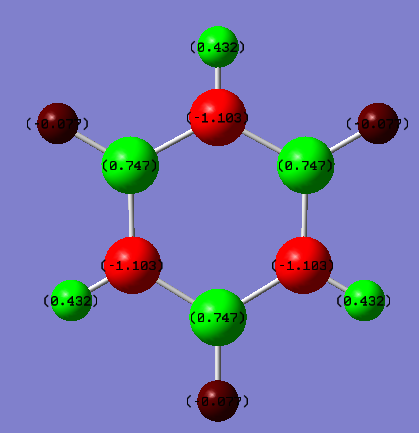
Borazine vs Benzene Charge Analysis
| Benzene | Borazine |
|---|---|
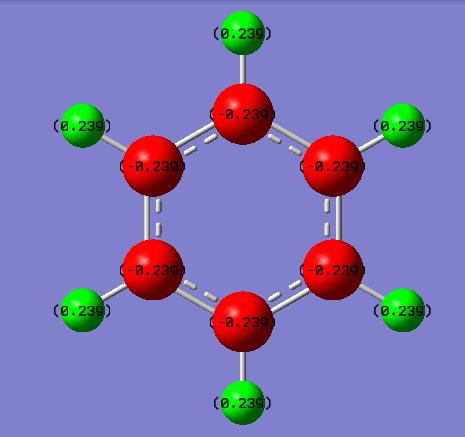 |
|
Ng611 (talk) 16:39, 21 May 2018 (BST) Remember to use the same color scale for both molecules!
| Atom | Charge (NBO) |
|---|---|
| Carbon | -0.239 |
| Hydrogen | 0.239 |
| Atom | Charge (NBO) |
|---|---|
| Nitrogen | -1.102 |
| Boron | 0.747 |
| Hydrogen attached to nitrogen | 0.432 |
| Hydrogen attached to boron | -0.077 |
From the pictures it can be seen that there is a greater charge distribution in borazine than in benzene due to the fact that there is a much greater difference in electronegativity between the atoms. In benzene there is very low localized charged on carbon and hydrogen atoms as the electronegativity of carbon is 2.55 and hydrogen is 2.2. This small difference in electronegativity results in a partial negative charge on the carbon atoms and a partial positive charge on the hydrogen. Borazine however has a much more apparent charge distribution due to the different electronegativities of nitrogen (3.04), boron (2.04) and hydrogen (2.2). This results in B-N bonds being polarised with the partial negative charge on the nitrogen, N-H bonds being polarised with the partial negative charge on the nitrogen but the B-H bonds are slightly polarised with the partial charge generated on the hydrogens.
Ng611 (talk) 16:42, 21 May 2018 (BST) Good explanation of the effects of electronegativity differences on the distribution of charge within the molecule. What is the sum of the partial charges? And how does symmetry affect the distribution of charge?
MO Comparison Benzene Vs Borazine
| Benzene | Borazine | Description |
|---|---|---|
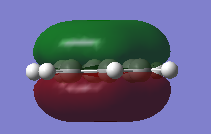 |
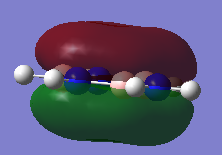 |
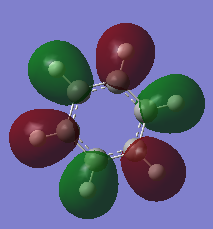
Comparison of MO17 of benzene and MO 17 of borazine. MO 17 for both molecules is a totally bonding with a p-orbitals containing electrons above and below the plane of the molecules. Both MOs are symmetric with contributions only from the p-orbitals which are perpendicular to the plane of the ring which form a cloud of electron density above and below the plane of the ring. This results in a node in the plane of the ring. By comparison of the energies of benzene MO17 (-0.35998 a.u.) compared to borazine (-0.36137 a.u.) it can be seen borazine is slightly more stable due to polarization of the bonds. |
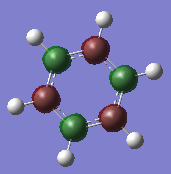 |
 |
Comparison of MO6 on benzene and MO 4 on borazine. Both involve 3 antibonding s-orbitals however benzene also involve 3 bonding s-orbitals whereas borazine does not. This is due to the fact that the boron 2s orbitals are much higher in energy than nitrogen and are too high in energy to interact therefore they form MOs where nitrogen orbitals do not contribute. Whereas in benzene all atoms in the ring are carbon therefore the 2s orbitals are all exactly the same energy and can interact to form this MO involving 3 bonding and 3 anti-bonding orbitals. This can also be seen in the energy of the MOs where in benzene it is -10.18685 a.u. wheres in borazine it is -6.74685 a.u. showing that the boron atoms are higher in energy than even carbon. Ng611 (talk) 16:43, 21 May 2018 (BST) I'd say that these are too AO like and deep in energy to be of any interest. |
 |
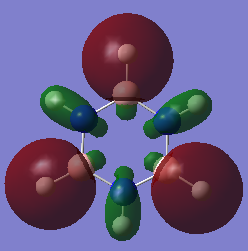 |
Comparison of MO13 on benzene and MO4 on borazine. Both sets of orbitals involve bonding interactions the C-H which involves some 2s and some 2p character of the carbon atoms interacting with the 1s orbital of the hydrogen atom. Similarly with borazine there is bonding interactions between the B-H and N-H atoms cause by overlap of 2s and 2p orbitals on nitrogen / boron and 1s orbital on hydrogen. However as can be seen the orbital interactions with respect to their neighbours are out of phase resulting in an anti-bonding interaction between adjacent C-H or N-H/B-H MOs. The resulting MOs have the energies -0.45822 a.u. and -0.38632 a.u. for benzene and borazine respectively. |
Concept of Aromaticity
Kekule first proposed the idea of what the structure of benzene may be after having a dream of a snake eating its tail. Since then it has been developed and the structure of benzene has been determined. Since than Huckel defined aromaticity as that the number of electrons the system contains must equal 4n+2; where n = the number of electrons perpendicular to the plane of the ring, this value can only equal integers including 0. The molecules must be planar and have a contiguous cyclic array of p-orbitals perpendicular to the plane of the ring. It is also possible to form anti-aromatic compounds which contain 4n electrons. Aromaticity provides additional stability to the structure whereas anti-aromaticity provides additional instability to the structure.
Aromatic compounds have special properties such as equal bond lengths within the ring caused by a conjugated system of double bonds which can resonate and additional stabilisation which is also attributed to the ability of the systems to resonate. Additionally to these in NMR measurements of aromatic compounds there is often very messy multiplets observed in the spectrum due to complicated coupling of the hydrogen atoms as well further to this they are quite deshielded due to the ring current effect caused by the conjugated system. https://onlinelibrary.wiley.com/doi/epdf/10.1002/chem.200700250
Basic views of orbital combinations such as the linear combination of atomic orbitals often are a good match to the molecular orbitals which are calculated by GaussView however can also vary drastically. Often the π-orbitals formed through both the linear combination of atomic orbitals and GaussView match each other quite well especially for highly symmetric systems. However, as the symmetry decreases and the bonds formed change to Ϭ-bonds the linear combination of atomic orbitals often becomes less accurate. In addition to this the linear combination of atomic orbitals considers generally which atoms are more polar than others and roughly what distortions may look like whereas when computed by GaussView these are much more accurate and smaller contributions are considered which would be overlooked in the linear combination of atomic orbitals giving a more accurate representation.
The concept of overlapping Pz orbitals is not the best description of aromaticity as, as mentioned above, distortions in orbital shape due to polarity will not be considered and different contributions to the different molecular orbitals (such as π-bonding and π*-antibonding) may not be considered. Whilst okay for benzene as all atoms of the ring are carbon and so the atomic orbitals may closely resemble Pz orbitals for borazine the different electronegativities is likely to result in difference in contributions to each of the molecular orbitals with boron atomic orbitals contributing more to π*-molecular orbitals and nitrogen orbitals contributing more to π-molecular orbitals.
Ng611 (talk) 16:48, 21 May 2018 (BST) A good overall report. You should be more careful in your MO analysis and select orbitals that you think will be important in dictating the chemistry of the molecule. Your discussion on aromaticity was good -- some thoughts on how the modern coneptual model of aromaticity differs from Huckel's model would improve the section further.