MRD:zz3116
1
What value do the different components of the gradient of the potential energy surface have at a minimum and at a transition structure? Briefly explain how minima and transition structures can be distinguished using the curvature of the potential energy surface.
The gradients in different directions at minimum and at transition state are all 0. Saddle point fits the situation: the second differentiation is smaller than 0 in one direction, and larger than 0 in the other.
Mm10114 (talk) 16:09, 7 June 2018 (BST) These are valid observations. However, in which direction is the second differentiation smaller than 0 and larger than 0 at the saddle point? You need to explain (surely not any random directions).
2
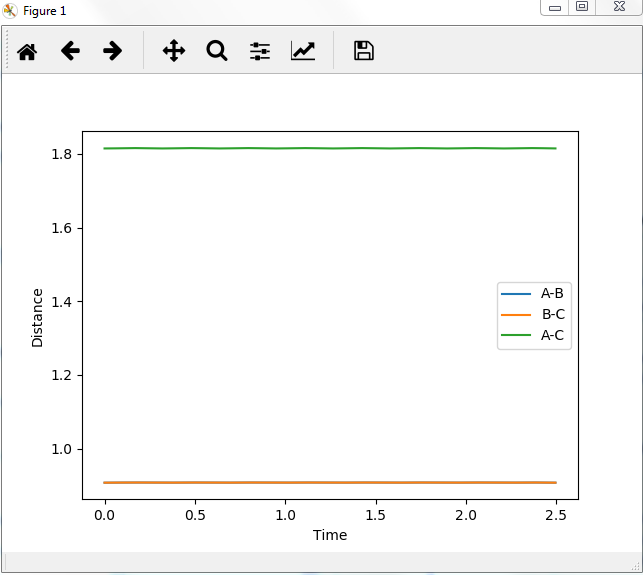
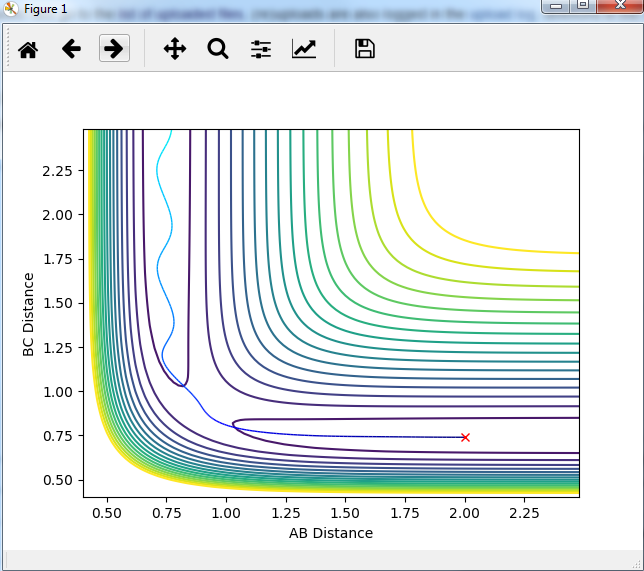
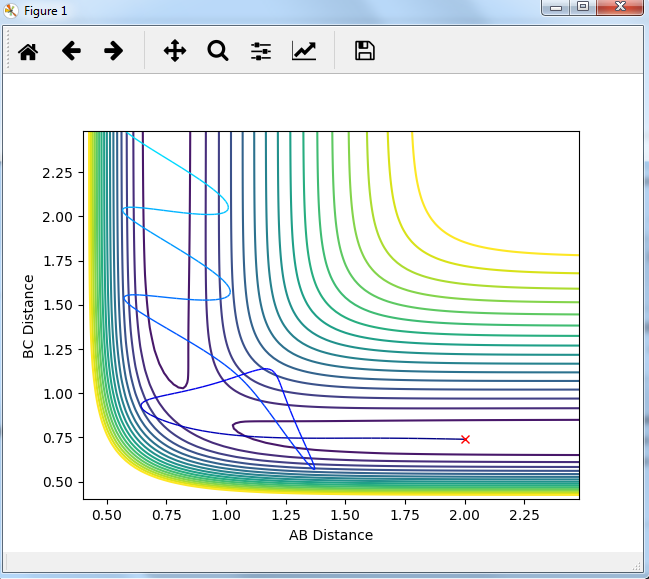
Report your best estimate of the transition state position (rts) and explain your reasoning illustrating it with a “Internuclear Distances vs Time” plot for a relevant trajectory.
rts=0.9075 , as can be seen ,flat lines means no vibrations.
Mm10114 (talk) 16:11, 7 June 2018 (BST) Your rts estimate is correct. However, you need to explain what these flat lines mean. You need to be more descriptive in your answers, showing the understanding, describing which line gives a value for what etc.
3
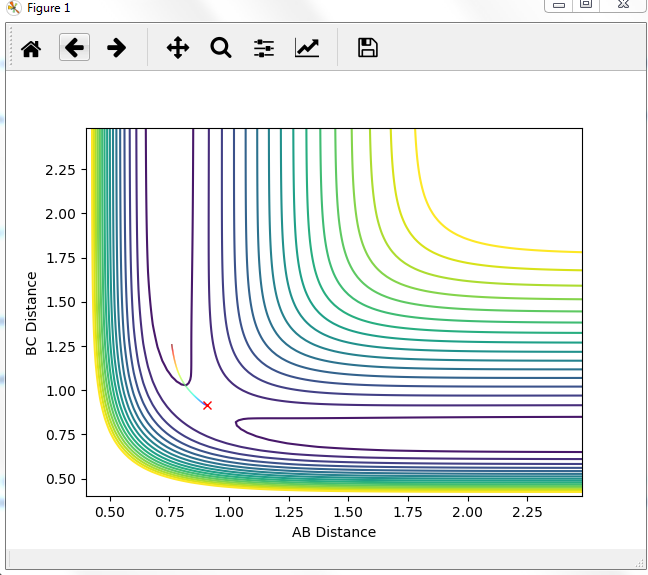
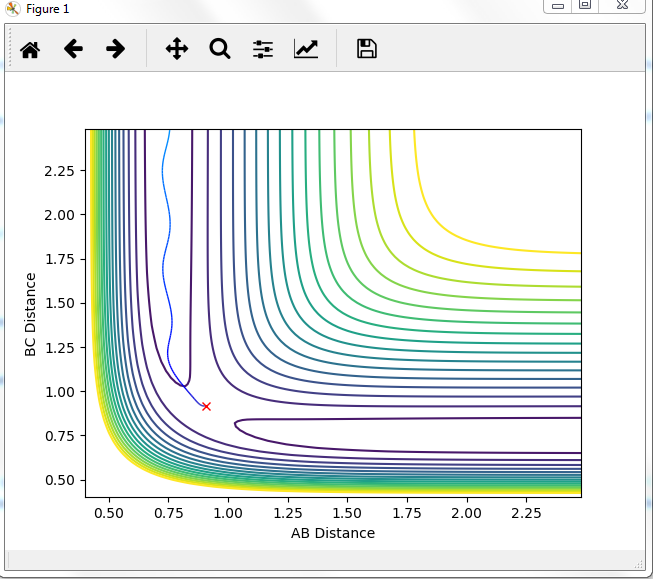
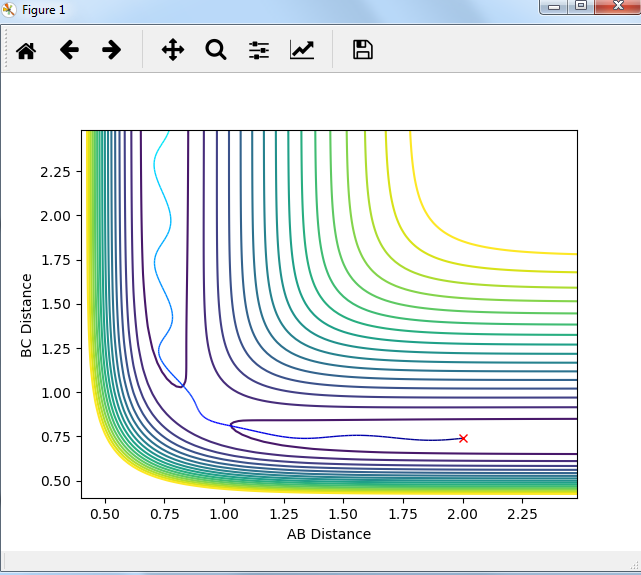
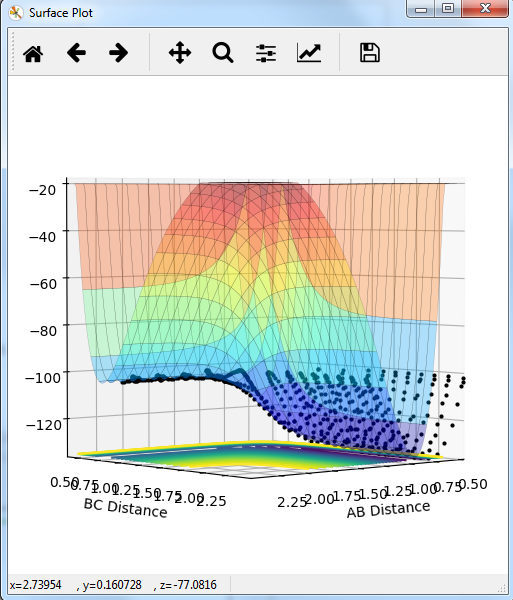
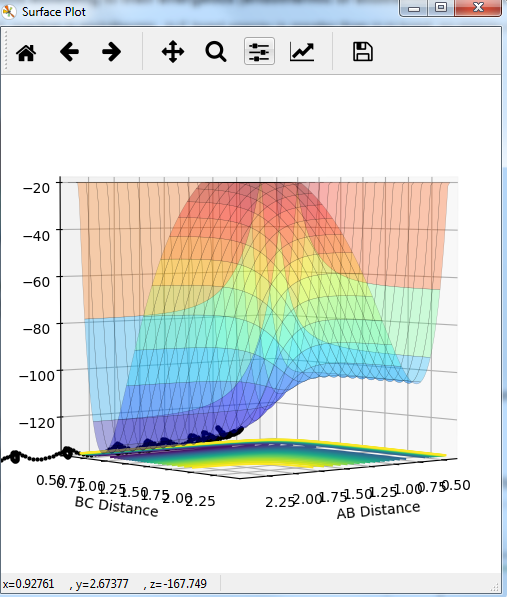
Comment on how the mep and the trajectory you just calculated differ.
MEP graph:
DYNAMIC graph:
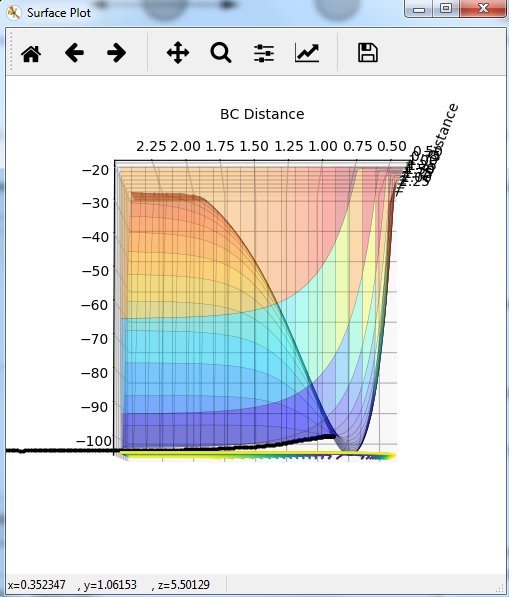
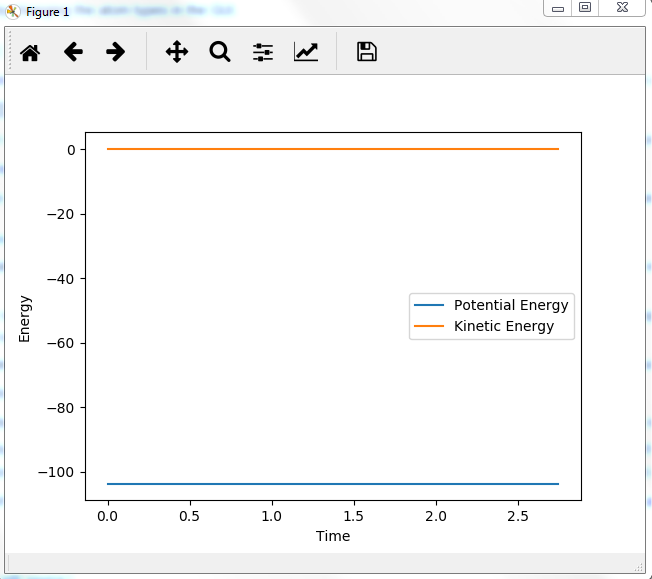
Potential energy graph:
the reaction goes towards the product. MEP means the lowest point in the oscillating energy in reaction path. We did not see any oscillations in the graph. The trajectory shows the actual energy changes in the reaction including any oscillations. P1=P2=0 means no initial kinetic energy. HB-HC distance is longer than that of HA-HB gives initial potential energy for the molecule. therefore, HB-HC bond oscillates along the potential energy surface.
Mm10114 (talk) 16:17, 7 June 2018 (BST) Why is the reaction going towards the products? ("product"? are you sure is there only one?) You need to explain why is it that we see no oscillation in MEP. Overall, your answer is sufficient, but it is very scarce. Some more in-depth explanation would be necessary here (cause -> effect), not just stating what is in the graphs, also you need to use the same references/naming for the bond distances as in the plots (AB/BC, not HA/HB/HC, or you need to explain how these relate to these on the plots).
4
Complete the table by adding a column with the total energy, and another column reporting if the trajectory is reactive or unreactive. For each set of initial conditions, provide a plot of the trajectory and a small description for what happens along the trajectory.
Mm10114 (talk) 16:19, 7 June 2018 (BST) Your answers are sufficient. The purpose of this exercise is to compare different initial conditions for p1 and p2, thus also making comments why in some cases these are reactive or not.
5
State what are the main assumptions of Transition State Theory. Given the results you have obtained, how will Transition State Theory predictions for reaction rate values compare with experimental values?
(You are quoting this from somewhere. You must cite your sources, otherwise you are incurring into plagiarism. João (talk) 12:40, 2 June 2018 (BST))
Transition state theory assumes:
1.Quantum-tunneling effects are assumed negligible
2.The born-oppenheimer approximation is invoked
3.the energy of atoms in the reactant is considered as Boltzman distributed
4.Once the system attains the transition state, the reaction would not go back to the reactant side again.
Comparison of experiment value and the TST:
1.in the actual experiment, the reaction can go back to the reactant partially.but the TST theory says the system would not reenter the reactant region after reaching the transition state. thus the experimental rate of reaction would be slower than the theoretical value.
2.the quantum tunneling takes place in the actual experiment. the energy of an atom decreases over the barrier rather than bounce. actual energy of each atom may not reach so high as the theory.
Exercise 2
Classify the F + H2 and H + HF reactions according to their energetics (endothermic or exothermic). How does this relate to the bond strength of the chemical species involved?
potential surface of F+HH reaction:
potential surface of H+HF reaction:
F+H2 reaction is exothermic, and H+HF reaction is endothermic. HF bond energy is stronger than HH bond as the dissociation energy of HF is larger than that of HH. HF is more stable.
7
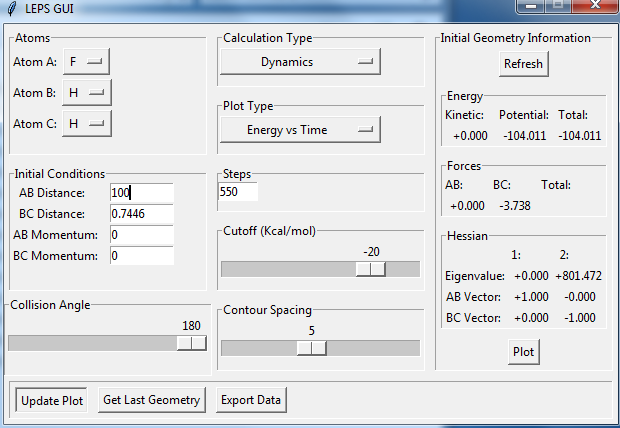
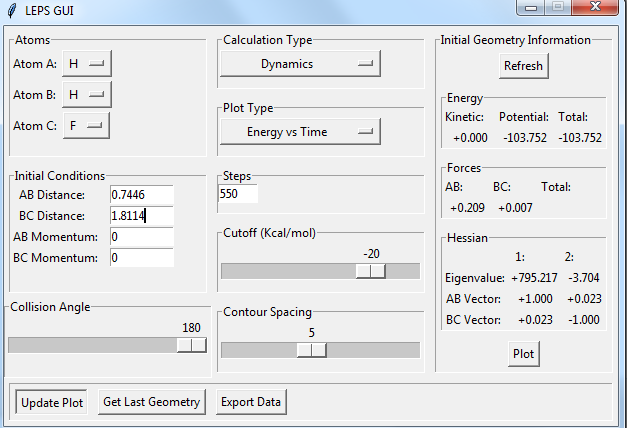
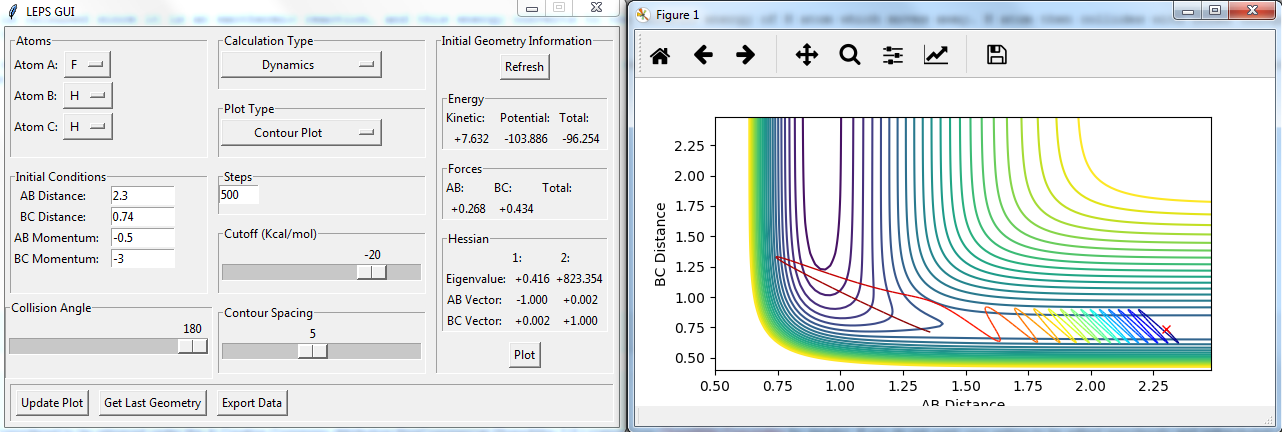
Locate the approximate position of the transition state.
the position of transition state is r(FH)=1.8114, r(HH)=0.7445. there are no vibrations at the transition state.
Mm10114 (talk) 16:23, 7 June 2018 (BST) How did you locate this transition state? Was it a lucky guess?
8
Report the activation energy for both reactions.
the reaction energy of F +H2 at transition state:
the reaction energy of F+H2 :
Ea of F+H2 =-103.752-(-104.011)=+0.259 kcal/mol.
the reaction of H+HF at transition state:
Ea of H+HF=-103.752-(-133.756)=30.004 Kcal/mol.
Mm10114 (talk) 16:28, 7 June 2018 (BST) Again, your values are correct. But, you need to be much more verbal about how you've obtained them. The initial conditions do not tell me much, without the appropriate justification and additional plots for distances or potential energy surfaces.
9
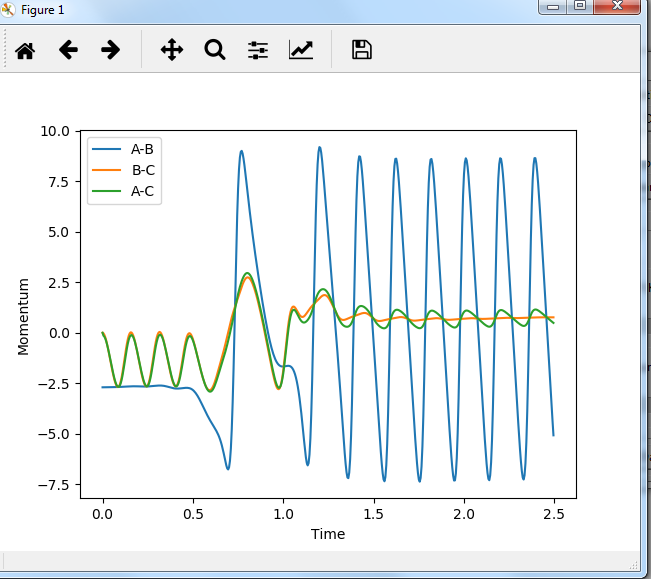
In light of the fact that energy is conserved, discuss the mechanism of release of the reaction energy. How could this be confirmed experimentally?
Initial conditions:
Animation:
Internuclear momenta vs time:
after the formation of HF bond, energy is released since it is an exothermic reaction, and this energy converts to the kinetic energy of H atom which moves away. H atom then collides with other things. Therefore, kinetic energy is converted to thermal energy. this can be confirmed by measuring the temperature.
Mm10114 (talk) 16:38, 7 June 2018 (BST) Very good observations. However, "H atom then collides with other things"? What other things? You need to be specific.
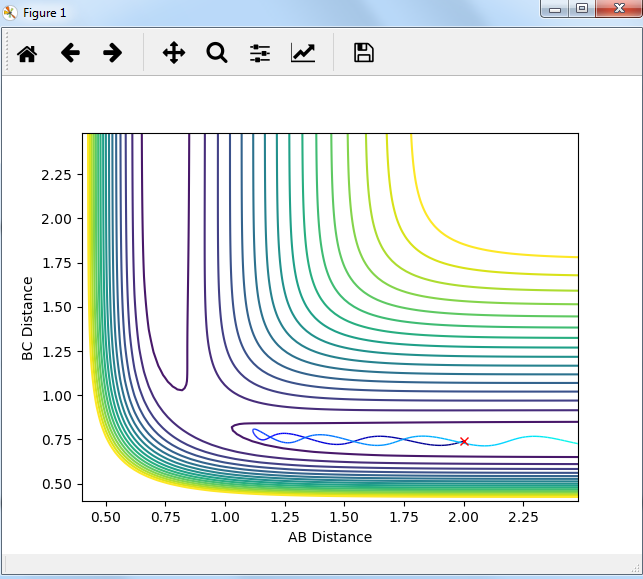
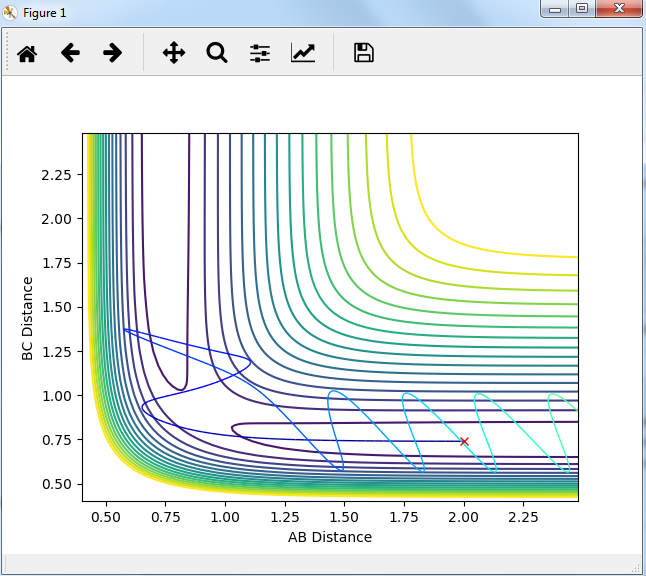
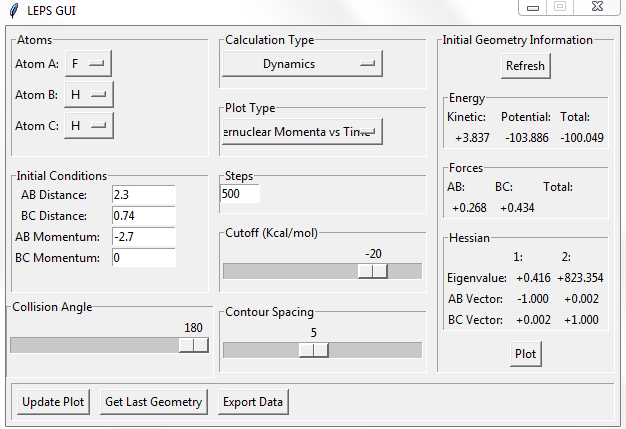
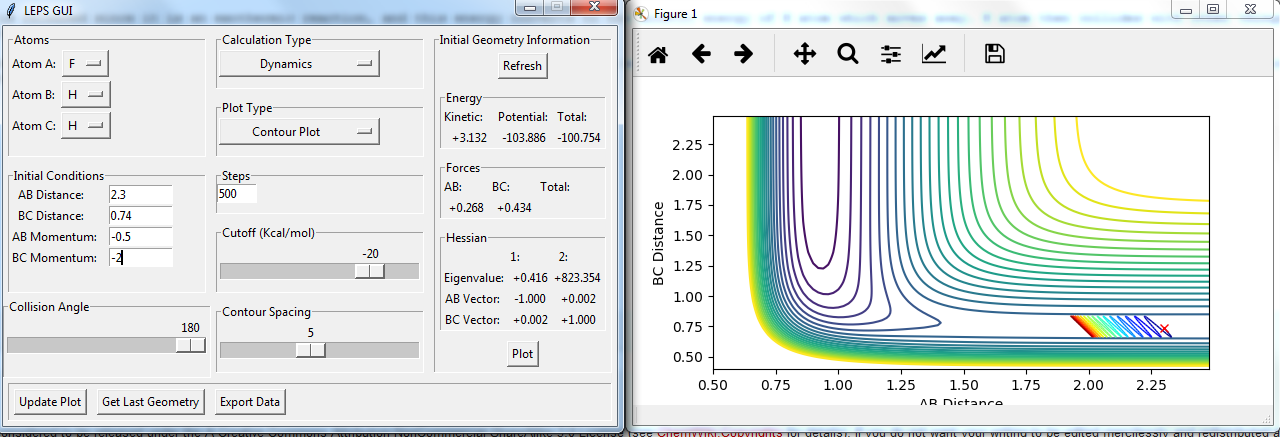
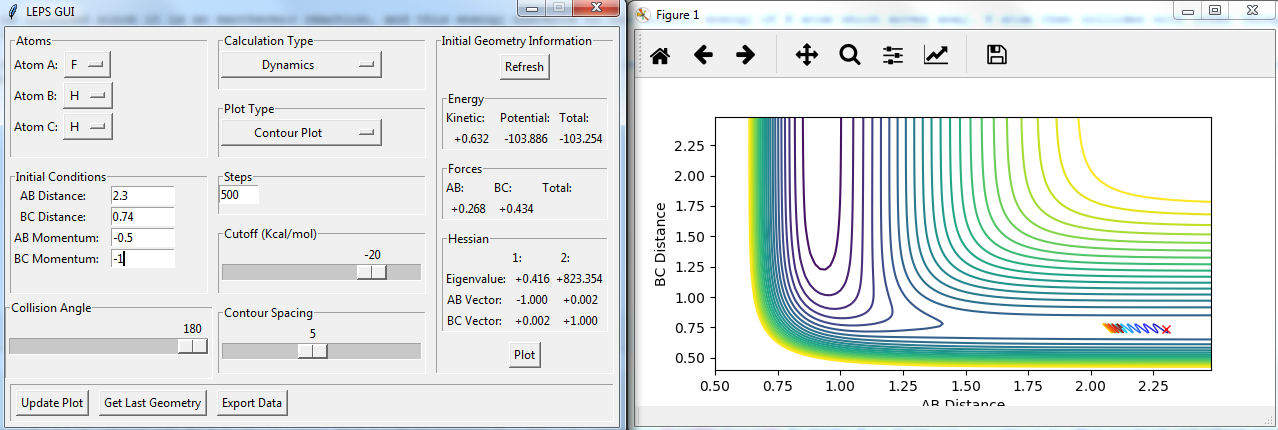
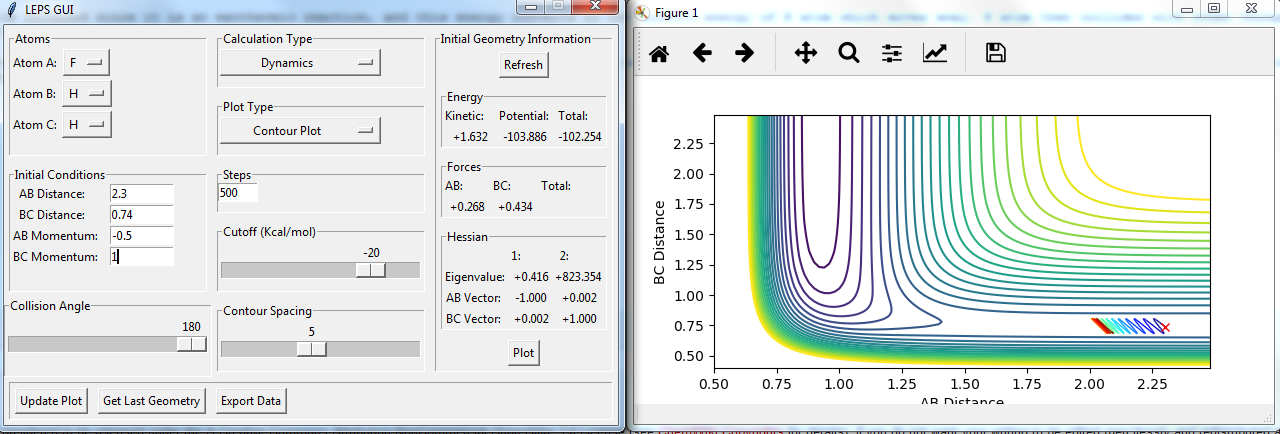
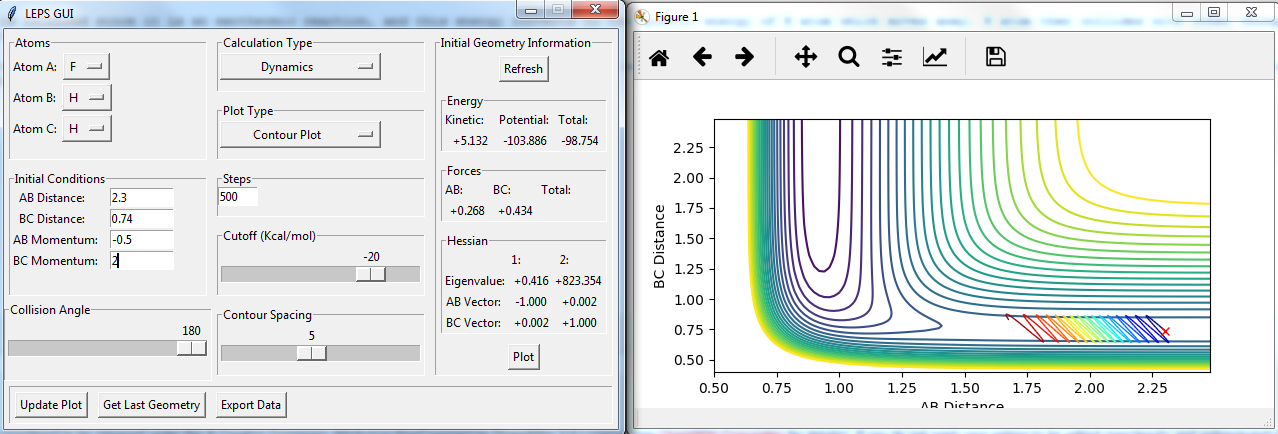
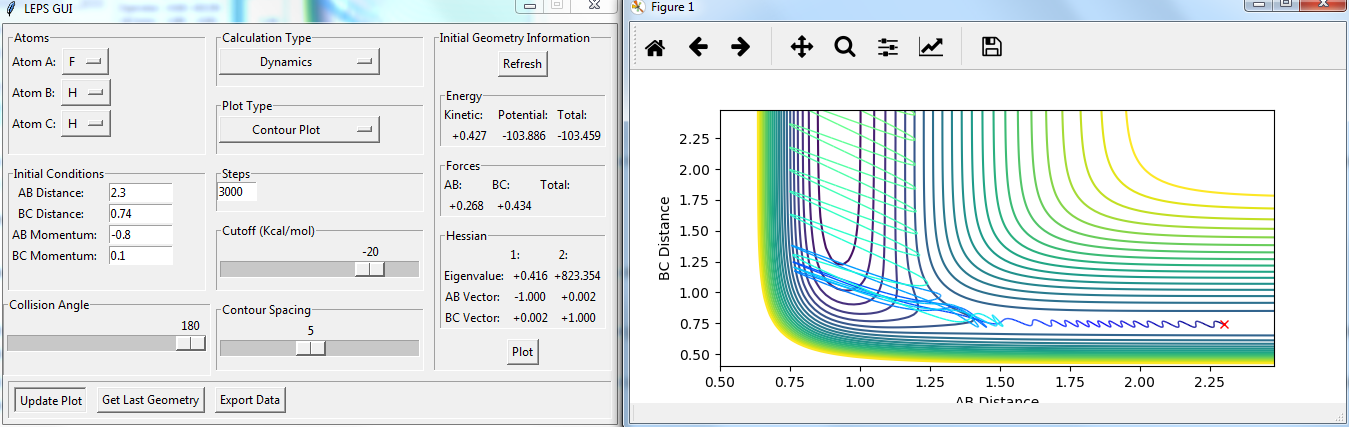
Setup a calculation starting on the side of the reactants of F + H2, at the bottom of the well rHH = 0.74, with a momentum pFH = -0.5, and explore several values of pHH in the range -3 to 3 (explore values also close to these limits). What do you observe?
figures with changing P2:
The momenta in the reaction provide main energy to activation. when the momentum of BC is set -3 or 3, it is reactive, otherwise it's not.
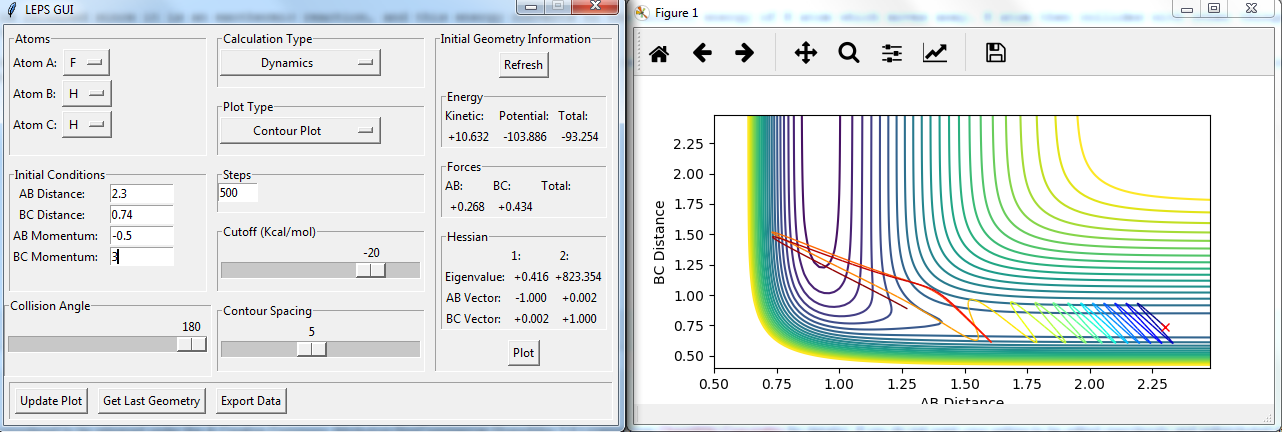
For the same initial position, increase slightly the momentum pFH = -0.8, and considerably reduce the overall energy of the system by reducing the momentum pHH = 0.1. What do you observe now?
the trajectory recrosses in the reaction but forms products finally. so it is reactive.
10
Discuss how the distribution of energy between different modes (translation and vibration) affect the efficiency of the reaction, and how this is influenced by the position of the transition state. F+H2 is exothermic with an early barrier, the translation has effect but vibration does not. For the early transition state, the vibration can provide the energy to get to the transition state, but it leads that reaction can recross the reactant region rapidly. H+HF is endothermic with a late barrier, the vibration has effect but transition does not.
Mm10114 (talk) 16:41, 7 June 2018 (BST) This is good. Have you come to these conclusions by yourself, or are you citing here Polanyi's rules? At the end, you made a mistake by saying transition instead of 'translation'. Be more careful.