MRD:zx2015y2MRD
Molecular Reaction Dynamics: H + H2 system
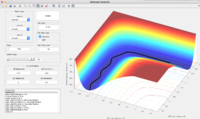
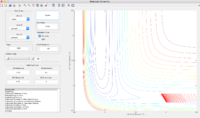
Gradient of the PES
The total gradient of the potential energy surface at a minimum and at a transition structure have value of 0. The second derivative of the potential energy surface can be calculated at two points and the minimum should have a positive second derivative value while the transition structure has a negative second derivative value.
(This is not necessarily true. The TS is the maximum along one axis and a minimum along another.Lt912 (talk) 08:22, 9 June 2017 (BST))
The transition state position
The best estimation for the transition state position was determined at rTS = 0.90775 Å . At rTS, the potential energy reaches minimum and the distances between each two of atom do not change any more.

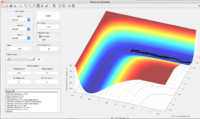
Dynamics and the minimum energy path(mep)
Internuclear Distances vs Time ( r1= rTS+δ, r2 = rTS)
In both dynamics and mep calculation, r1 increases and r2 decreases to a constant value. But in dynamics calculation, this process is much quicker than in mep calculation. For the same 1000 steps calculation, the r1 in dynamics calculation reaches approximately 18 Å while in mep calculation it only goes to approximately 1.4 Å.
(This is not the only difference between an MEP and dynamics calculation, check the lab script. Lt912 (talk) 08:23, 9 June 2017 (BST))
Internuclear Momenta vs Time ( r1= rTS+δ, r2 = rTS)
In the dynamics calculation, p1 goes up quickly until reaches constant at 2.5 and p2 drops first then rises up and oscillates between 1 and 1.5. In the mep calculation, the momenta remains 0 during the whole process.
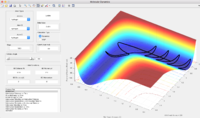
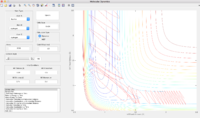
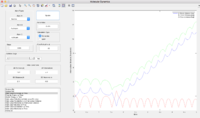
Reactive and unreactive trajectories (r1 = 0.74, r2 = 2.0)
Summery
Assumption on Transition State Theory
1. The motion of the atoms follow Newton's equations of motion.
2. The atoms must have enough energy to form transition state after collision.
(You are missing some. Lt912 (talk) 08:24, 9 June 2017 (BST))
Comparison with Experiment
The predicted reaction rate are expected to be faster than the experimental reaction rate. The reason for that was the collision angle was set to be 180 degrees which is not always the case in reality. The possibility to collide at right angle is low hence the experimental reaction rate would be lower than prediction.
Molecular Reaction Dynamics: F- H- H system
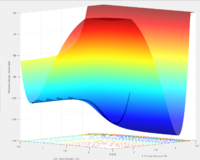
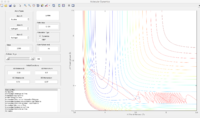
According to the PES graph, the potential energy of the system is much lower when fluorine atom is close to one of the hydrogen atom. Hence the F+ H2 reaction is exothermic while the HF+ H reaction is endothermic. The bond strength of HF bond is higher than H2 bond.
The transition state position is determined at rHF=1.81053 Å, and rHH=0.74535Å.
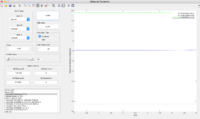
The energy of Transition state is determined to be -103.3kcal/mol.
F+ H2 Reaction
The energy of the reactant is -103.9kacl/mol.
Ea=-103.9-(-103.3)=-0.6kcal/mol.
HF+ H Reaction
The energy of the reactant is -133.7kcal/mol.
Ea=-133.7-(-103.3)=-30.4kcal/mol.
Reaction Dynamics
F+ H2 Reaction
The F+ H2 reaction is an exothermic reaction, energy released in the form of heat. To measure the energy released, the temperature change of the reaction mixture can be measured to determine the heat released.
The reactants of this reaction are set at the bottom of the well rHH=0.74 and pHF=-0.5. pHH is varied from -3 to 3. It was observed that when pHH is in the range of -2 to 1.4, the system is not reactive. In the momentum range of -3 to -2 and 1.4 to 3, some systems are reactive will the others are not.
When pHF is increased to -0.8, the system is reactive even when the pHH is as low as 0.1.
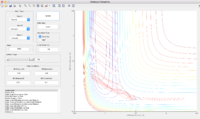
H+ HF Reaction

With low vibrational motion on the H- F bond and high value of pHH, the system is unreactive. By increase pHF and decrease pHH, the system becomes reactive again when the pHF is over 5.3 and pHH=-0.5.
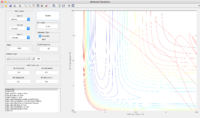
Conclusion
In the exothermic reaction, the energy barrier is low, the translational energy of the incoming atom dominates. An increase in translational energy can increase the efficiency of the system but changes in the vibrational energy of the system does not affect the efficiency much.
In the endothermic reaction, sufficient vibrational energy required to weaken the bond since the energy barrier is so high. In this case, vibrational energy of the system dominates.
(I think you could expand on this a little bit. Lt912 (talk) 08:26, 9 June 2017 (BST))