MRD:zl6217
Ng611 (talk) 21:44, 12 May 2019 (BST) Overall a good attempt at the report, although you made one or two major mistakes (for example, I would have expected you to realise that there is no physical meaning for a negative activation energy, and revisit your calculations accordingly). Other points of improvement are highlighted elsewhere. Otherwise, this was a good effort and a well presented piece of work.
H2 + H System:
Q1. On a potential energy surface diagram, how is the transition state mathematically defined? How can the transition state be identified, and how can it be distinguished from a local minimum of the potential energy surface?
- The transition state is recognized as the maximum of the minimum energy path. In terms of the mathematical expression, it's when ∂V(ri)/∂ri=0; In this case, (∂V(AB)/∂AB)*(∂V(BC)/∂BC)=0 on the potential energy surface diagram
- The second derivatives of ∂V(ri)/∂ri can be calculated to differentiate: when ∂V2(ri)/∂ri2 > 0, the trajectory is at the transition state; when ∂V2(ri)/∂ri2 <0, it is at a local minimum of the potential energy surface.
Ng611 (talk) 21:06, 12 May 2019 (BST) A good general explanation, but you need to define your co-ordinate system. What does r_i represent? Where do their basis vectors lie on the PES?
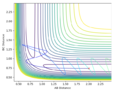
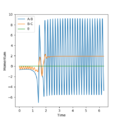
Q2. Report your best estimate of the transition state position (rts) and explain your reasoning illustrating it with a “Internuclear Distances vs Time” plot for a relevant trajectory.
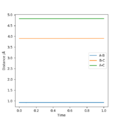
An original Interdistance vs Time diagram was observed. Because the transition state occurs when AB=BC, the interdistance of TS is around 0.907.
For H2 + H, since the atoms involved are all identical, AB and BC is going to be at the same distance in the transition state because the extent of the bonding forming and breaking is the same. Furthermore, since transition state is at the local maximum of the potential energy surface diagram, the kinetic energy of the state should be 0 (particles stop moving).
Several tests was run when p1=p2 and AB=BC=0.907/0.906/0.908/0.900; The transition state is confirmed to be AB=BC=0.907 since according to the animation and interdistance vs time diagram, the vibrations of the particles are at the minimal which means the potential energy reaches its highest point. Furthermore, the particles A and C stop oscillating and slide to the product.
Ng611 (talk) 21:27, 12 May 2019 (BST) Good, but this behaviour would also be the same if the particle was at a local minimum. Could you explain what you did to demonstrate that you had obtained a maximum?
Q3.Comment on how the mep and the trajectory you just calculated differ.
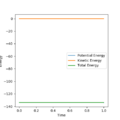
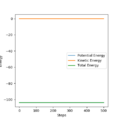
The mep: minimum energy path; the velocity is reset to zero after each time unit. Therefore, the oscillation of particle won't be shown on the trajectory; the conservation of energy and the inertia of particles was omitted
For mep calculation: trajectory simply follows the bottom of the energy valley floor to H1+ H2-H3 , there is a smooth increase and decrease of distance AB and BC; the total energy decrease because of the decrease in potential energy
For dynamics calculation: The BC distance decrease and fluctuate which indicate the oscillation and the total energy remains unchanged.
Ng611 (talk) 21:27, 12 May 2019 (BST) Good!
Q4. Complete the table above by adding the total energy, whether the trajectory is reactive or unreactive, and provide a plot of the trajectory and a small description for what happens along the trajectory. What can you conclude from the table?
| p1 | p2 | Etot | Reactive | Description of the dynamics |
|---|---|---|---|---|
| -1.25 | -2.5 | -99.018 | yes | trajectory simply follows the valley floor to H1+ H2-H3. The AB increases smoothly and BC decreases while fluctuating |
| -1.5 | -2.0 | -100.456 | no | p2 is decreased by 20%,B first approaches to C and move backward while the BC distance is still large. Meanwhile, AB distance fluctuating around a steady value. |
| -1.5 | -2.5 | -98.956 | yes | The p2 was increased again; the trajectory simply follows the valley floor to H1+ H2-H3. The AB increases smoothly and BC decreases while fluctuating |
| -2.5 | -5.0 | -84.956 | no | Both p1 and p2 were both increased for a significant extent: A crossing-transition state occurs: B first approached to C, BC bond was formed and oscillated about a fixed point for a short period and B approaches tp A again, form bond with A while C move backwards |
| -2.5 | -5.2 | -83.416 | yes | The p2 was further increased for a small amount: B first approached to C, BC bond was formed and oscillated about a fixed point for a short period. Afterwards, BC became longer and B move closer to A shortly then move back to C again. Eventually, BC bond was formed and oscillated forward to the right hand side while A moves away to the left hand side. |
Overall, the total energy of particles in all situations were above the activation energy of the reaction. This implies that having energy above Ea is not the only thing determine the reactivity of the collision, the relative value between p1 and p2 could be the other significant determinant. p1 and p2 might need to be in certain ratio that BC momenta is large enough to overcome momenta AB to form a new bond between BC after breaking of AB bond. For example, compared with the reactive situation in the third row, the reaction in the fourth row could not happen although both p1 and p2 were increased since the p1 was so large relative to p2 that after temporary bond forming between BC, AB bond was reformed and oscillated away from the center. The distribution of vibrational and transitional energy could also explain the reactivity shown in the table, which is shown in the diagrams.
Q5. State what are the main assumptions of Transition State Theory. Given the results you have obtained, how will Transition State Theory predictions for reaction rate values compare with experimental values?
The assumptions:
1. For the reactants, the atoms are all Boltzmann distributed, i.e. the state is thermally equilibrate.
2. If the system reaches the transition state and have a velocity towards product, the system will not move back to the initial reactant state.
3. The quantum-tunneling effects are omitted when analyzing the potential energy surface diagram.
4. Born-Oppenheimer approximation is applied.[1]
According to the table above, there is a possibility of crossing transition state in the reality. In comparison, the TST simplifies the rate constant calculation process by assuming all the transition state could be transferred into product successfully. Therefore, it is reasonable to conclude that the experimental value could be lower than the predicted rate values since there are unsuccessful transition because of the quantum tunneling effect.
Ng611 (talk) 21:30, 12 May 2019 (BST) Quantum tunnelling and TS recrossing are two separate phenomena (and indeed, they typically have the opposite effect to one and other). You are correct that TS theory overestimates the reaction rate, however.
F-H-H System
Q1. By inspecting the potential energy surfaces, classify the F + H2 and H + HF reactions according to their energetics (endothermic or exothermic). How does this relate to the bond strength of the chemical species involved?
According to the surface diagram:
H2 + F : exothermic
F + HF: endothermic
From the potential energy surface diagram: the potential energy of the system H2 + F is higher than that of H + HF.
From the left valley to the right valley: H2 + F reaction;
From the right valley to the left valley: H+ HF reaction.
HF is with larger bond strength compared with HH, therefore breaking HF absorbs higher energy than the energy released from bond forming of HH bond and vice versa. It is corresponding to the energetics of this two reaction extracted from the surface diagram.
Ng611 (talk) 21:31, 12 May 2019 (BST) Good!
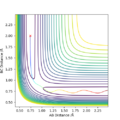
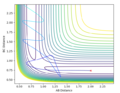
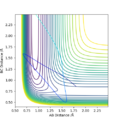
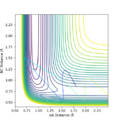
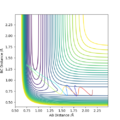
Q2. Locate the approximate position of the transition state
Where the vibration is at its minimal, kinetic energy approaches to zero and the state is at the maximum of the potential energy surface:
The transition state is where AB=1.845 and BC= 0.74. The BC was found to be similar as H-H bond, it is corresponding to the reaction scheme since H2 + F is closer to the TS.
Ng611 (talk) 21:32, 12 May 2019 (BST) I get what you mean, and you're absolutely right, but your explanation could have been a bit clearer.
Furthermore, the energy of the transition state is -103.747 kcal/mol.
Q3. Report the activation energy for both reactions.
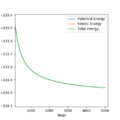
For F + H2: the activation energy = ETS - ER = -133.129 - (-103.747)= -29.38 kcal/mol
The ER was measured from zoom-in diagram of energy v.s. time after 5000 time units
For H + HF: the atoms H and F were exchanged to calculate the activation energy for the reversed process; The activation energy = ETS - ER = -133.459-(-133.78)=-0.321 kcal/mol
The difference between the two activation energy is reasonable since F + H2 is an exothermic reaction and its Ea supposes to be smaller than its reversed process. Furthermore, regarding to the similarity between the TS and F + H2, the small value of the activation energy could be reasonable.
Ng611 (talk) 21:35, 12 May 2019 (BST) These values are totally incorrect. Firstly, it is completely unreasonable to report a negative Ea value; this would imply that the TS lower in energy than the reactants and is there is no barrier to the reaction. This also makes it difficult to compare these values.
Q4. In light of the fact that energy is conserved, discuss the mechanism of release of the reaction energy. Explain how this could be confirmed experimentally.
With AB= 2, BC= 0.74, p1=-0.7 and p2 = -0.1, the F + H2 tends to be reactive.
Regarding to the conservation of energy, the energy released via an exothermic reaction could be converted into heat. Experimentally, it could be confirmed as increasing in temperature.( calorimetry )
Ng611 (talk) 21:40, 12 May 2019 (BST) Calorimetry would give you the aggregate change in energy (if done accurately), but would not tell you how that liberated energy is distributed (i.e.: how much is in translations vs. vibrations).
Q5. Discuss how the distribution of energy between different modes (translation and vibration) affect the efficiency of the reaction, and how this is influenced by the position of the transition state.]
The Polanyi's empirical rules states that the late-barrier reaction (endothermic) is mainly drawn by vibrational energy while the transitional energy is less efficient. Meanwhile, it's reversed for the early-barrier reaction which is exothermic.[2]
For endothermic reaction H + HF:
The collision was reactive when there is high transitional energy and low vibrational energy. However, when there is a relatively low transitional energy and higher vibrational energy ,the trajectory became unreactive. The experimental result is contradict to the Polanyi's empirical rules.
In case of high transitional energy, the trajectory is reactive, late barrier reaction
In case of high vibrational energy, the trajectory is inreactive, late barrier reaction
For exothermic reaction F + H2:
The experimental results are also contradict to the Polanyi's rules:
In case of high transitional energy, the trajectory is inreactive, early barrier reaction:
In case of high vibrational energy, the trajectory is still reactive, early barrier reaction:
In conclusion, the Polanyi's empirical rules does not fit in all situations to predict the reactivity.
Ng611 (talk) 21:41, 12 May 2019 (BST) This is true-- they are heuristics after all. Most likely, the F+H2 reaction is so facile that the reaction will go ahead regardless.
Reference:
- Dmitry Murzin, 2005,Tapio Salmi, Catalytic Kinetics, Elsevier B.V.,Finland.
- Dynamics of the Reaction of Methane with Chlorine Atom on an Accurate Potential energy Surface. G.Czako,J.M. Bowman, 2011, Science,334(6054), 343-346, [assessed 03/05/2019]