MRD:zcj17mrdwiki
BLUE QUESTIONS
On a potential energy surface diagram, how is the transition state mathematically defined? How can the transition state be identified, and how can it be distinguished from a local minimum of the potential energy surface?
Both ∂V(r1)/∂r1 and ∂V(r2)/∂r2 will equal 0 and the second derivatives of each variable will have opposing signs.
If you imagine placing a ball near the local minimum, it will roll towards the minimum, whereas if you place a ball at the saddle point, it will roll towards either the reactants or the products, depending on what side the ball was originally placed on.
In the next HHH system example, what if you place the ball at r1=r2 = x, where x is between 0.7-0.8?. What do you see? Does it fall either reactants or the products?Sw2711 (talk) 23:22, 9 May 2019 (BST)
Report your best estimate of the transition state position (rts) and explain your reasoning illustrating it with a “Internuclear Distances vs Time” plot for a relevant trajectory.
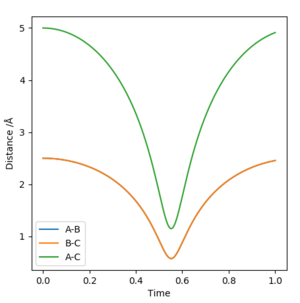
r(ts)=0.57 Å
You need to double check your number. I shall expect a straight line in distance vs time graph. Do you know why? Sw2711 (talk) 23:22, 9 May 2019 (BST) The transition state position will be the smallest internuclear distance. This will be the minimum in the graph of internuclear distance vs time.
Comment on how the mep and the trajectory you just calculated differ.
In the mep, the atoms have no vibrational energy after the collision, whereas in dynamics, the two bonded atoms vibrate. Furthermore, in the dynamics calculation, the internuclear distances are larger, due to the higher velocity. What do you mean no vibrational energy after the collision. Then what about before the collision? Also, why does the MEP doesn't have velocity?
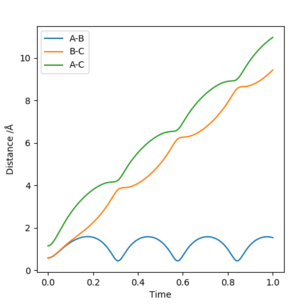
Complete the table above by adding the total energy, whether the trajectory is reactive or unreactive, and provide a plot of the trajectory and a small description for what happens along the trajectory. What can you conclude from the table?
| p1 | p2 | Etot | Reactive? | Description of the dynamics |
|---|---|---|---|---|
| -1.25 | -2.5 | -99.199 | Yes | AB and C approach each other and the bond between A and B breaks, while a bond between B and C forms, with some vibrational energy. The products move off in opposite directions. |
| -1.5 | -2.0 | -100.456 | No | No bonds are broken or formed, and after the collision both reactants move off with a lot of vibrational energy Has the system reached the TS barrier? Sw2711 (talk) |
| -1.5 | -2.5 | -98.956 | Yes | AB and C approach each other and the bond between A and B breaks, while a bond between B and C forms, with more vibrational energy. The products move off in opposite directions. |
| -2.5 | -5.0 | -84.956 | No | AB and C approach each other and the bond between A and B breaks. B weakly bonds with C, however the molecule has such a large amount of vibrational energy that the newly formed molecule breaks again. This process repeats between A and B, before B once again bonds with C and moves off with a large amount of vibrational energy |
| -2.5 | -5.2 | -83.416 | Yes | AB and C approach each other and the bond between A and B breaks. B weakly bonds with C, however the molecule has such a large amount of vibrational energy that the newly formed molecule breaks again. This process repeats between A and B, before B once again bonds with C and moves off with a large amount of vibrational energy |
In all of the cases you described, did they pass the TS? And how did they cross?
State what are the main assumptions of Transition State Theory. Given the results you have obtained, how will Transition State Theory predictions for reaction rate values compare with experimental values?
Transition State Theory assumes that once reactants have passed the transition state, they will always go on to form products. However, as the final entries to the previous question show, this is not always the case. Therefore TS theory will overestimate the rate of reaction.
Good. But is it the only assumption in the TS theory?Sw2711 (talk) 23:22, 9 May 2019 (BST)
By inspecting the potential energy surfaces, classify the F + H2 and H + HF reactions according to their energetics (endothermic or exothermic). How does this relate to the bond strength of the chemical species involved?
The forward reaction, F + H2 is exothermic, making the reverse reaction, HF + H, endothermic. This means the H-F bond is much stronger than the H-H bond. formed will be This means, according to Hammond's Postulate, the transition state will be closer to the reactants.
Yes, what could you tell from a energy surface plot? What would the energy surface appear like for endo/exothermic reactions?Sw2711 (talk) 23:22, 9 May 2019 (BST)
Locate the approximate position of the transition state.
AB distance = 1.809 Å
BC distance = 0.750 Å
How did you get those values, how do you confirm it? Sw2711 (talk) 23:22, 9 May 2019 (BST) Report the activation energy for both reactions.
For the F + H2 reaction, the activation energy is approximately 140 kJ/mol For the HF + H, the activation energy is approximately 1 kJ/mol 142 1.60 kJ/mol
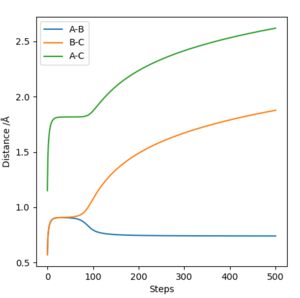
Same as above. Sw2711 (talk) 23:22, 9 May 2019 (BST) Identify a set of initial conditions that results in a reactive trajectory for the F + H2, and look at the “Animation” and “Momenta vs Time”.In light of the fact that energy is conserved, discuss the mechanism of release of the reaction energy. Explain how this could be confirmed experimentally.
Set of initial conditions:
| AB Distance | BC Distance | pAB | pBC |
|---|---|---|---|
| 1.5 | 0.5 | -0.1 | 0.5 |
The reaction energy could be released in several different ways. This includes increasing the velocity of the particles or giving them more vibrational or rotational energy. You still haven’t told me how this could be confirmed experimentally? And where is your answer for the last question? Sw2711 (talk) 23:22, 9 May 2019 (BST)
